三种ADAMTS13去整合素样结构域突变致蛋白功能缺陷及其与血栓关联的研究
Three disintegrin-like domain mutations of ADAMTS13: functional deficiency and association with thrombosis
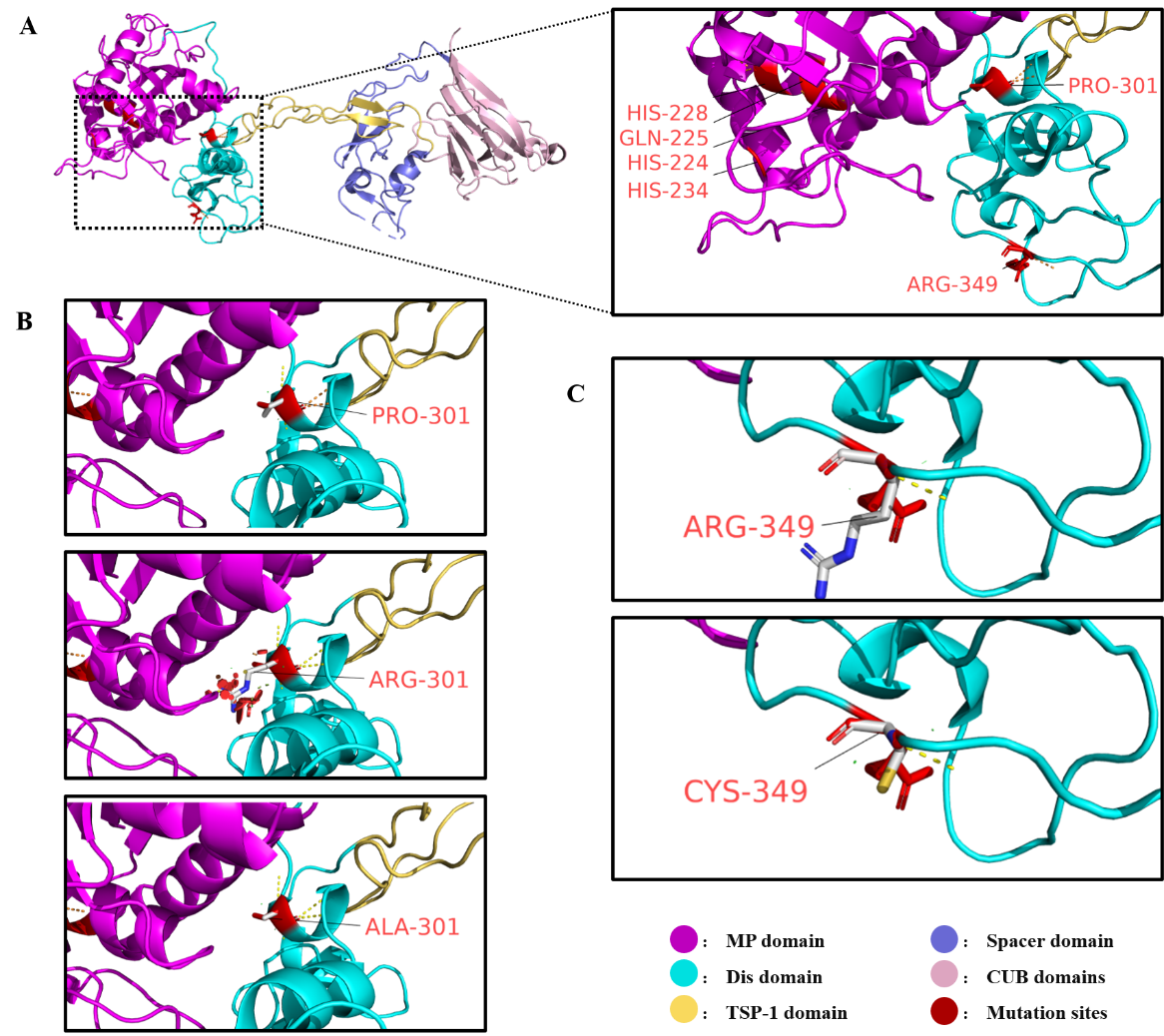
A:野生型ADAMTS13蛋白质结构,金属蛋白酶结构域上的催化活性中心(HIS-224、GLN-225、HIS-228、HIS-234)和本文研究的突变位点(PRO-301、ARG-349)以红色标出。B:野生型ADAMTS13氨基酸残基PRO-301与突变型ADAMTS13中氨基酸残基ALA-301、ARG-301的结构差异对比。突变后的ARG-301残基周围的红色圆盘表示发生的空间位阻效应。C:野生型ADAMTS13氨基酸残基ARG-349与突变型ADAMTS13中氨基酸残基CYS-349的结构差异对比。
A: The structure of wild-type ADAMTS13 protein, with the catalytic active site (HIS-224, GLN-225, HIS-228, HIS-234) in the metalloprotease domain and the studied variant sites (PRO-301, ARG-349) highlighted in red. B: Structural comparison between wild-type ADAMTS13 residue PRO-301 and mutated residues ALA-301 and ARG-301 in ADAMTS13. The red disks surrounding the ARG-301 residue represent steric hindrance caused by the variant. C: Structural comparison between wild-type ADAMTS13 residue ARG-349 and mutated residue CYS-349 in ADAMTS13.