


Journal of Tissue Engineering and Reconstructive Surgery >
Evaluation of Three Kinds of Skin Repair Materials on the Effectiveness of Skin Injury Repair in Diabetic Rats
Received date: 2020-04-10
Revised date: 2020-05-06
Online published: 2020-06-26
Objective To evaluate the effectiveness of collagen, carboxymethyl chitosan and gelatin bioactive glass in repairing the skin injury of diabetic rats.
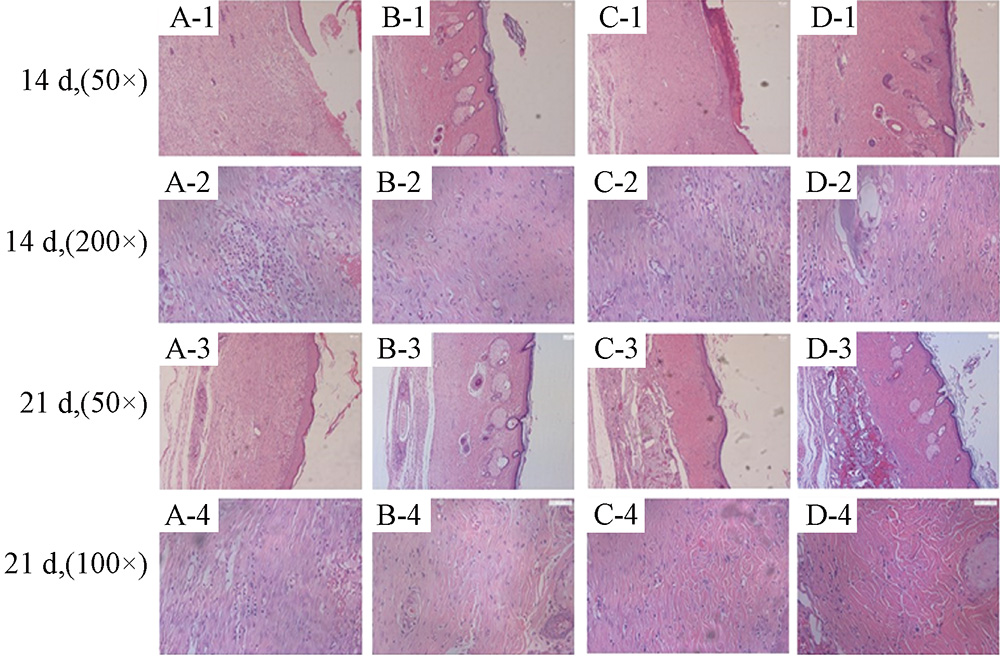
Methods The best skin injury model of diabetic rats was established by injecting different concentration of STZ solution in single time and excising the whole skin of different sizes in the back of SD male rats respectively. Three kinds of skin dressings were attached directly to the injured area and covered with sterile dressings in order to promote skin healing. The general wound recovery was observed and wound healing rate of each group was calculated. HE staining was used to observe the recovery of wound in different periods.
Results A single intraperitoneal injection of 75 mg/Kg STZ solution and the construction of 2 cm×2 cm back skin injury could establish the best skin injury model of diabetic rats. Collagen and gelatin bioactive glass promoted skin healing to a certain extent, and the healing rate was statistically different from the blank control.
Conclusion Collagen and gelatin bioactive glass have therapeutic effect on skin injury of diabetic rats, carboxymethyl chitosan only has hemostatic effect in early stage.
Key words: Diabetes; Skin injury; Collagen; Carboxymethyl chitosan; Gelatin bioactive glass
Qianqian HAN, Mingyue QU, Bin XUE, Chunren WANG . Evaluation of Three Kinds of Skin Repair Materials on the Effectiveness of Skin Injury Repair in Diabetic Rats[J]. Journal of Tissue Engineering and Reconstructive Surgery, 2020 , 16(3) : 232 -236 . DOI: 10.3969/j.issn.1673-0364.2020.03.015
| [1] | Sorg H, Tilkorn DJ, Hager S , et al. Skin wound healing: an update on the current knowledge and concepts[J]. Eur Surg Res, 2017,58(1-2):81-94. |
| [2] | Law JX, Chowdhury SR, Aminuddin BS , et al. Role of plasma-derived fibrin on keratinocyte and fibroblast wound healing[J]. Cell Tissue Bank, 2017,18(4):585-595. |
| [3] | Grazul-Bilska AT, Johnson ML, Bilski JJ , et al. Wound healing: the role of growth factors[J]. Drugs Today (Barc), 2003,39(10):787-800. |
| [4] | Mahdavian Delavary B, van der Veer WM, van Egmond M , et al. Macrophages in skin injury and repair[J]. Immunobiology, 2011,216(7):753-762. |
| [5] | Nissen NN, Polverini PJ, Koch AE , et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing[J]. Am J Pathol, 1998,152(6):1445-1452. |
| [6] | Stadelmann WK, Digenis AG, Tobin GR . Physiology and healing dynamics of chronic cutaneous wounds[J]. Am J Surg, 1998,176(2A Suppl):26s-38s. |
| [7] | Gholipourmalekabadi M, Sapru S, Samadikuchaksaraei A , et al. Silk fibroin for skin injury repair: Where do things stand?[J] Adv Drug Deliv Rev, 2019,[Epub ahead of print]. |
| [8] | Wang J, Chen Y, Zhou G , et al. Polydopamine-coated Antheraea pernyi (A.pernyi) silk fibroin films promote cell adhesion and wound healing in skin tissue repair[J]. ACS Appl Mater Interfaces, 2019,11(38):34736-34743. |
| [9] | Malgarim Cordenonsi L, Faccendini A, Rossi S , et al. Platelet lysate loaded electrospun scaffolds: Effect of nanofiber types on wound healing[J]. Eur J Pharm Biopharm, 2019,142:247-257. |
| [10] | Yang W, Xu H, Lan Y , et al. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair[J]. Int J Biol Macromol, 2019,130:58-67. |
| [11] | Zahiri M, Khanmohammadi M, Goodarzi A , et al. Encapsulation of curcumin loaded chitosan nanoparticle within poly (ε-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution[J]. Int J Biol Macromol, 2019,[Epub ahead of print]. |
| [12] | Huang L, Zhu Z, Wu D , et al. Antibacterial poly (ethylene glycol) diacrylate/chitosan hydrogels enhance mechanical adhesiveness and promote skin regeneration[J]. Carbohydr Polym, 2019,225:115110. |
| [13] | Xiao M, Gao L, Chandrasekaran AR , et al. Bio-functional G-molecular hydrogels for accelerated wound healing[J]. Mater Sci Eng C Mater Biol Appl, 2019,105:110067. |
| [14] | Veeruraj A, Liu L, Zheng J , et al. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications[J]. Mater Sci Eng C Mater Biol Appl, 2019,95:29-42. |
| [15] | Yao CH, Chen KY, Chen YS , et al. Lithospermi radix extract-containing bilayer nanofiber scaffold for promoting wound healing in a rat model[J]. Mater Sci Eng C Mater Biol Appl, 2019,96:850-858. |
| [16] | Ding H, Fu XL, Miao WW , et al. Efficacy of autologous platelet-rich gel for diabetic foot wound healing: a meta-analysis of 15 randomized controlled trials[J]. Adv Wound Care (New Rochelle), 2019,8(5):195-207. |
| [17] | Lavery LA, Davis KE, Berriman SJ , et al. WHS guidelines update: Diabetic foot ulcer treatment guidelines[J]. Wound Repair Regen, 2016,24(1):112-126. |
| [18] | Zhang P, Lu J, Jing Y , et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis[J]. Ann Med, 2017,49(2):106-116. |
| [19] | Morbach S, Furchert H, Gr?blinghoff U , et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade[J]. Diabetes Care, 2012,35(10):2021-2027. |
| [20] | Rakieten N, Rakieten ML, Nadkarni MV . Studies on the diabetogenic action of streptozotocin (NSC-37917)[J]. Cancer Chemother Rep, 1963,29:91-98. |
| [21] | Takitani K, Inoue K, Koh M , et al. α-Tocopherol status and altered expression of α-tocopherol-related proteins in streptozotocin-induced type 1 diabetes in rat models[J]. J Nutr Sci Vitaminol (Tokyo), 2014,60(6):380-386. |
| [22] | Gao T, Jiao Y, Liu Y , et al. Protective effects of konjac and inulin extracts on type 1 and type 2 diabetes[J]. J Diabetes Res, 2019,2019:3872182. |
| [23] | Aghanoori MR, Smith DR, Shariati-Ievari S , et al. Insulin-like growth factor-1 activates AMPK to augment mitochondrial function and correct neuronal metabolism in sensory neurons in type 1 diabetes[J]. Mol Metab, 2019,20:149-165. |
| [24] | Sedlak L, Wojnar W, Zych M , et al. Effect of resveratrol, a dietary-derived polyphenol, on the oxidative stress and polyol pathway in the lens of rats with streptozotocin-induced diabetes[J]. Nutrients, 2018,10(10):E1423. |
| [25] | Wu KK, Huan Y . Diabetic atherosclerosis mouse models[J]. Atherosclerosis, 2007,191(2):241-249. |
| [26] | Paik SG, Michelis MA, Kim YT , et al. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens[J]. Diabetes, 1982,31(8 Pt 1):724-729. |
| [27] | Boateng JS, Matthews KH, Stevens HN , et al. Wound healing dressings and drug delivery systems: a review[J]. J Pharm Sci, 2008,97(8):2892-2923. |
| [28] | Dragostin OM, Samal SK, Dash M , et al. New antimicrobial chitosan derivatives for wound dressing applications[J]. Carbohydr Polym, 2016,141:28-40. |
| [29] | Gillette RL, Swaim SF, Sartin EA , et al. Effects of a bioactive glass on healing of closed skin wounds in dogs[J]. Am J Vet Res, 2001,62(7):1149-1153. |
/
| 〈 |
|
〉 |