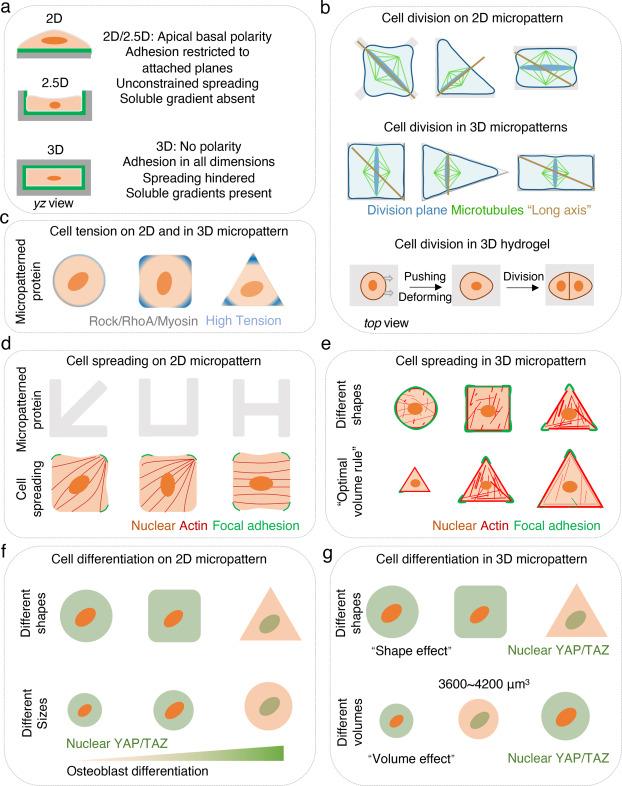
At the single-cell level and collectively, cell tension is consistently highest at the corners or edges of micropatterns, both in 2D and 3D
10,18,23,87,88,89,90 (
Fig. 2c). In 2D, the adhesive geometry of cells plays an important role in cell tension enrichment and cell spreading.
13,43,44,45,91 Single cells plated on 2D micropatterns interact with the ECM through integrins and the formation of focal adhesions (FAs).
12,13,14 This occurs predominantly at the cell periphery (circular)
13 or cell corners (square) and is regulated by the micropattern shape
9,10,12,14 (
Fig. 2d). Single cells on round islands generate tensile forces along the circumference without a preferred direction, whereas cells on square islands tend to exhibit the highest tension at the corners.
87,90 FAs are associated with the termini of contractile actin and myosin filaments, which generates tension in response to external and internal traction forces.
10,12,14 Consequently, cells develop their stress fiber and tension distributions according to the geometry of the adhesive area.
10,18,23,24,87,89,90 The most prominent contractile tension is generated along cell edges, particularly the concave edge of a non-adhesive region, where the stress fibers are anchored to ECM by FAs.
9,10,12 Similarly, multiple cells on micropatterns also demonstrate the strongest stress fibers and contractility in the cells located at the edges.
23,92 Through micro- and nano-patterning, researchers discovered that cell is affected by spatial constraints and adhesion. Although both approaches yield similar cell morphologies, they initiate distinct signaling pathways and influence cellular functions differently: nanopatterning via nano-grooves amplifies intracellular force and fosters osteogenic differentiation through the RhoA/ROCK pathway, while micropatterning through micro-grooves induces pseudopodia formation.
93 Overall, cells sense the geometrical constraints produced by micropatterned ECM causing cytoskeletal filament reorganizations and actomyosin contractility transformation.