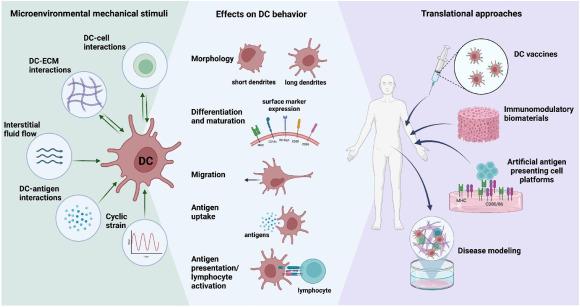
Migration is also an important part of DC function, whether migrating through the TME to capture tumor antigens, or migrating to lymphoid tissues following its maturation to activate the adaptive immune response. Rho-family GTPases are known to play a central role in DC migration: specifically, Rho activation causes DC extensions to retract while Rac activation causes the extensions to protrude further.
117 Following their uptake, processing of antigens, and maturation, DCs become highly migratory and enter the lymphatic vessels through which they migrate to the lymph nodes where they activate T cells.
118,119 Their migration to and through lymphatic vessels is known to be guided by chemokine signaling involving CCR7 receptors and chemokines such as CCL19 and CCL21.
120,121,122 Substrate stiffness has been shown to influence the expression of CCR7 thereby altering their guided migration by CCL21 signaling, suggesting that tissue stiffness may influence the ability of mDCs to reach the lymphatic vessels.
99 mDC migration to the lymphatic vessels is also facilitated by their initial docking to the lymphatic endothelium receptor LYVE-1 via its ligand hyaluronan which are binded to CD44 on the surface of mDCs.
123,124 mDCs enter the lymphatic vessels through specialized button-like flap valves of about 2-3 μm in diameter.
125 Increasing evidence also suggests that the flow of interstitial fluid towards the lymphatic vessels may affect the signaling induced by chemokines and ultimately plays a role in guiding DCs towards the lymphatic vessels, perhaps through a mechanism term autologous chemotaxis.
126,127,128 Autologous chemotaxis is a process, whereby cells secrete chemokines and interstitial fluid drainage towards lymphatics generates a local chemokine gradient through which cells perform directed migration towards lymphatics.
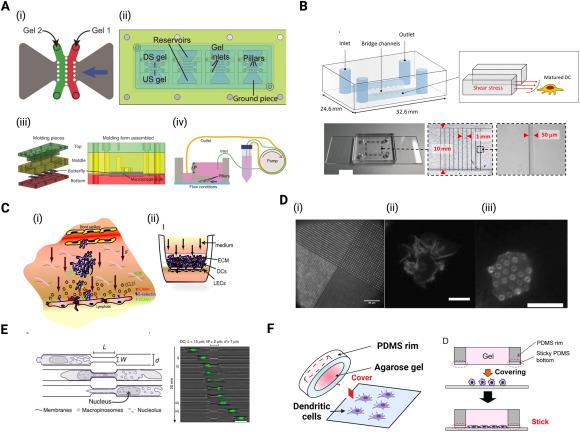
129 This has been shown in MDA-MB-231 breast cancer cell line, which possesses CCR7 and autologously secretes CCL19, and exhibited directed migration in response to flow (
Fig. 5A).
93 To mimic the intensity of shear stress generated in inflamed tissues such as tumors, Kang et al. developed a microfluidic channel with which they generated shear stress with intensity within 0.2-0.6 dyne/cm
2, similar to that in inflamed tissues (
Fig. 5B).
85 Using this microfluidic model, they found that shear stress did not alter the speed of migrating DCs but influenced their direction of migration,
85 possibly through the aforementioned autologous chemotaxis. Another factor that influences the DC migration is their binding capabilities to the ECM and to other cells such as endothelial cells which are found in the lining of blood and lymphatic vessels. Compared to monocytes and iDCs, mDCs have been found to possess lower binding capability to vascular endothelial cells which is in part due to their downregulation of CD11a, CD106 (VCAM-1) and CD54 (ICAM-1)
130,131 which are required for adhesion. Additionally, the membrane of mDCs possess relatively excess negative charges which causes them to experience powerful repulsive forces from negatively charged endothelial cells.
131 Overall, the decreased binding of mDCs to vascular endothelial cells may facilitate their migration through lymphatic vessels. Furthermore, the pumping activity that causes flow of lymph may also lead to either increase or decrease in rate of DC transit depending on the velocity of lymph flow.
128 Moreover, transmural flow has been shown to trigger lymphatic endothelium to increase expression of DC chemoattractant molecule CCL21 and adhesion molecule ICAM-1 and decrease expression of lymphatic junctional adhesion molecules PECAM-1 and vascular endothelial (VE)-cadherin (
Fig. 5C).
94 Combined, these events increase lymphatic flow, thereby facilitating DC transmigration into lymphatic vessels and transit across lymphatic endothelium.
94 Upon reaching the lymph node, mDCs travel along the fibroblastic reticular cells (FRC) to reach T cell zones where they can locate and prime T cell response.
132 Their migration along the FRC network is dependent on the interaction of the C-type lectin receptor, CLEC-2 which is expressed by DCs with podoplanin (PDPN) expressed on FRCs.
133 The activation of CLEC-2 by PDPN triggers DC spreading through the downregulation of Rho activity and myosin light-chain phosphorylation and causes the formation of F-actin-rich protrusions via Vav signaling and Rac1 activation.
133 Ultimately, this leads to a rearrangement of the actin cytoskeleton of DCs and promotes their migration along the FRC network.
133