1. Introduction
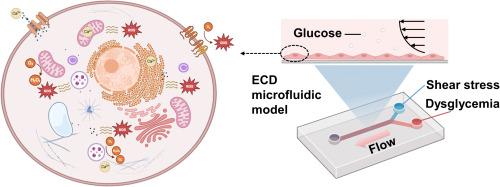
Fig. 1. Microfluidic investigation for SS-mediated repair of dysglycemia-induced ECD. Employing microfluidic technology, an ECD microfluidic model is constructed to achieve synergistic effects of accurate pulsatile SS loading and dysglycemia stimulating. This model allows the investigation of the interplay between cellular ROS and Ca2+ signals, aiming to seek interventions that utilize appropriate SS to repair ECD. Picture created with BioRender.com. |
2. ECD caused by dysglycemia
2.1. Hyperglycemia
2.2. Hypoglycemia
2.3. GF
3. SS repairs ECD induced by dysglycemia
3.1. SS and ECs
3.2. SS as a treatment strategy
4. Microfluidic technology for loading SS and simulating blood glucose environment
4.1. Microfluidic technology
4.2. SS generation using microfluidic technology
4.2.1. Various SS stimuli
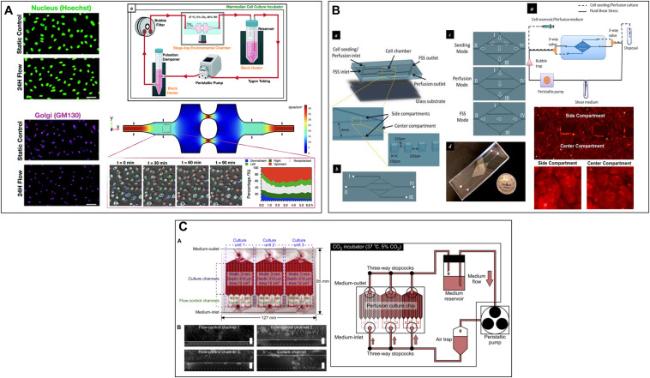
Fig. 2. Microfluidic device that can exert SS on ECs. (A). A novel microfluidic multimodal SS generator for studying endothelial cell polarization and orientation to flow (Adapted from reference 78 with permission). (B) A diamond-shaped microfluidic device that simultaneously generates high and low SS for revealing stress amplitude-dependent and load duration-dependent cellular responses (Adapted from reference 79 with permission). (C) A microfluidic perfusion cell culture chip for analyzing the influence of different SS on vascular endothelial function (Adapted from reference 80 with permission). |
Table 1. Summary of microfluidic systems for recapitulating SS of in vivo flow environment. |
| Microfluidic device | SS | Stimulation time | Main characterization | Reference |
|---|---|---|---|---|
| Microfluidic SS gradients generator | 0∼86 dyn/cm2 | 24 h | •Cell morphology | 81 |
| 20−150 dyn/cm2 | 48 h | •Cell growth | 82 | |
| 1∼9 dyn/cm2 | 1 h | •Cell adhesion | 83 | |
| 4∼40 dyn/cm2 | 24 h | •Golgi-nucleus polarization and orientation | 78 | |
| 0.01-0.09 Pa | 12 h | •Cell morphology •Cell apoptosis •Cellular ROS levels | 84 | |
| 0∼2.2 Pa | 24 h | •Cell morphology •Inflammatory responses | 85 | |
| Wall SS signal changes as a square wave with a period of 60 s between 1.875 and 2.813 Pa | 45min | •Intracellular Ca2+ dynamics | 86 | |
| •Constant shear: 17.6 dyn/cm2 •Stepped shear: 23.7-35-58.1 dyn/cm2 | 14 h | •Cell morphology •VE-cadherin expression | 87 | |
| 1∼1.7 Pa | 30min each day, three days | •Cell viability •Cell morphology | 88 | |
| A micro-vasculature-on-a-chip device | •96 h at 0.4 Pa •96 h at 0.4 Pa + 1 h at 0.08 Pa •96 h at 0.4 Pa + 6 h at 0.08 Pa •96 h at 0.08 Pa | •Actin skeleton | 53 | |
| 0, 0.02, 0.37, 1.12, 1.86 dyn/cm2 | 4 days | •Cell morphology •Mechanical properties | 89 | |
| Microfluidic cardiac profile generator | •7-11 dyn/cm2 •9-17 dyn/cm2 •13-27 dyn/cm2 •26-56 dyn/cm2 With 0.8, 1.2, 2 Hz frequencies | 48 h | •Cell morphology | 90 |
| Estimated maximum and average SS: 0.42 Pa, 0.09 Pa | 48 h | •Cell morphology | 91 | |
| Wall SS waveforms at rest, low, middle, and high intensities of exercise states | 420s | •Intracellular Ca2+ response | 21,92 | |
| Pulsatile SS: 4 dyn/cm2 & 20 dyn/cm2; 1 Hz & 2 Hz | 24 h | •Actin filaments' orientation •β-catenin distribution •Nucleus size and shape | 93 | |
| Others | Disturbed flow | 16 h | •Actin stress fibers' orientation and nuclear shape | 94 |
| Oscillatory flow | •Dye visualization | 95 |
4.2.2. Accurate pulsatile SS stimulus
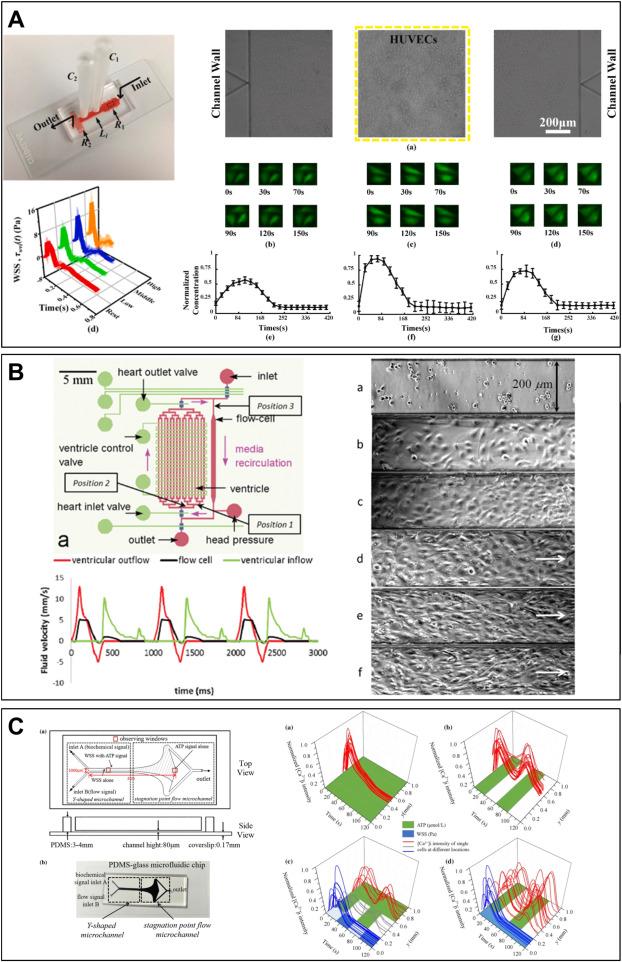
Fig. 3. Using a microfluidic system to construct accurate in vivo SS waveforms acting on ECs. (A) A microfluidic system for precise and stable construction of physiological blood pressure and SS to study the Ca2+ response of ECs (Adapted from reference 21 with permission). (B) A microfluidic device to simulate microcirculation for studying endothelial cell response to pulsatile SS (Adapted from reference 91 with permission). (C) A microfluidic device for synergizing SS and ATP signaling for studying their key roles in HUVECs signal transduction (Adapted from reference 96 with permission). |
4.3. Synergistic effects of pulsatile SS and dysglycemia
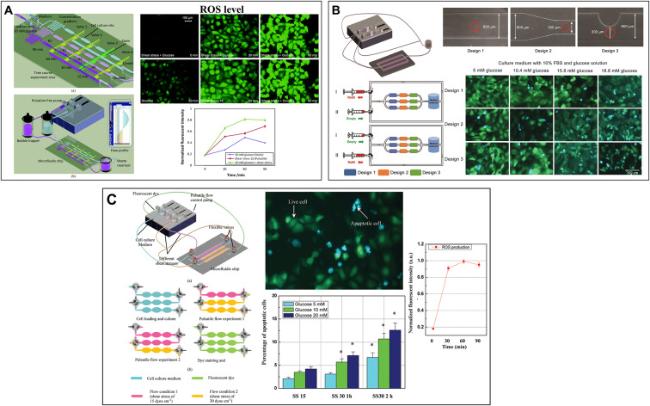
Fig. 4. Microfluidic devices that synergize SS and dysglycemia. (A) A hemodynamic chip system for studying endothelial cell ROS production and apoptosis under different pulsatile SS and glucose concentrations (Adapted from reference 22 with permission). (B) A hemodynamic chip system for simulating the microenvironment of pathological blood (Adapted from reference 99 with permission). (C) Integration of microfluidic chip system and FRET biosensor to study endothelial cell apoptosis (Adapted from reference 98 with permission). |
Table 2. Microfluidic systems for simulating the synergistic effect of SS and blood glucose. |
| Microfluidic system | SS | Glucose concentrations | Stimulation time | Main characterization | Reference |
|---|---|---|---|---|---|
| Wall plate microfluidic device system | 1 dyn/cm2 | Hyperglycemia: 80 and 200 mg/dl | 60 and 180 min | •Glycocalyx thickness •ROS generation | 100 |
| Endothelial cell culture model | Normal flow: frequency: 80 bmp & amplitude: 35 dyn/cm2; Disturbed flow | Normal glucose: 1 g/L; High glucose: 4.5 g/L | 24 h | •Cellular organization and actin alignment •eNOS phosphorylation (p-eNOS) •Akt and p-Akt •p-FAK | 59 |
| Hemodynamic lab-on-a-chip system | 70 bmp & average SS: 15 dyn/cm2; 110bmp & average SS: 23.6 dyn/cm2; | Control group: 5 mM; Experimental group: DM patients' plasma | 8 h | •Cell apoptpsis | 99 |
| Hemodynamic lab-on-a-chip system | 70 bmp & 15 dyn/cm2 (SS15); 140 bmp & 30 dyn/cm2 (SS30); | 5 mM; 10 mM; 20 mM | •12 h SS15 •1 h SS30 + 11 h SS15 •2 h SS30 + 10 h SS15 | •Cell apoptosis and necrosis | 99 |
| Hemodynamic lab-on-a-chip system | Constant SS: 30 dyn/cm2; SS15; SS30 | 5.5 mM; 10 mM; 20 mM | •0∼120min; •0∼3 h | •ROS production •Mitochondrial morphology | 22 |
| 3D microfluidic device | 0.4 Pa | 5.5 mM; 30 mM | 7 days | •Actin alignment •Cell apoptosis •Glycocalyx thickness | 101 |
5. Crucial roles of ROS and Ca2+
5.1. Imbalance of ROS and Ca2+ induced by dysglycemia
5.2. Ca2+ and ROS signals during repair of SS using microfluidic technology
Fig. 5. Hemodynamic microenvironment in arterial ECs and Ca2+ and ROS-related signaling pathways. |