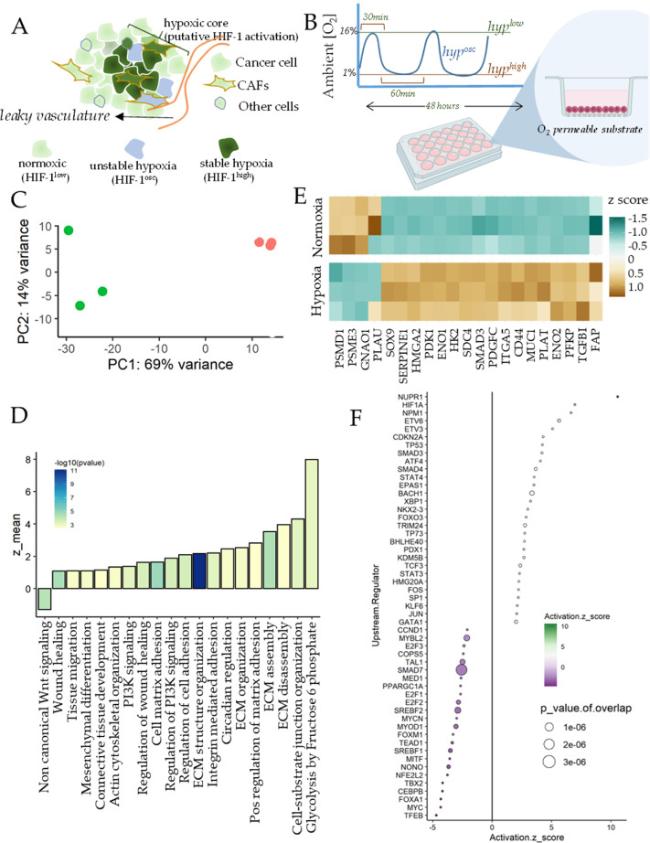
Principal Component Analysis showed a large separation in gene expression for normoxia and hypoxia samples (
Fig. 1C). To assess the broad contours of gene expression changes, we identified the key gene ontologies (GOs) activated by hypoxia. We found many GOs significantly activated in hypoxia were related to regulation of extracellular matrix assembly, and organization, actin cytoskeletal reorganization, tissue migration, and wound healing (
Fig. 1D). In addition, we also found activation of glycolytic processes and circadian regulation, which are well known to be regulated by HIF-1.
14 Other notable GOs were those associated with cell-matrix adhesion, including integrin activation, cell-matrix adhesion, and cell-substrate junction organization. Crucially, non-canonical Wnt signaling was downregulated by hypoxia in breast CAFs. Overall, hypoxia appeared to directly regulate many matricellular and biomechanical pathways associated with wound healing, and stromal response to cancer dissemination (
Fig. 1D). We selected the genes prominently contributing to activation of these pathways from the top activating sets, finding key genes responsible for fibroblast activation, wound healing, and matrix interaction, as well as those related to glycolysis (
Fig. 1E). These included genes encoding key components of the Transforming Growth Factor beta (TGFβ) pathway, involved in myofibroblast transformation of fibroblasts, including transcriptional co-regulator Smad3, TGFβ-1 ligand itself, and TGFBI encoding the TGFβ -inducible protein. In addition, we found other key genes associated with CAFs interaction with matrix to be increased by hypoxia, including FAP encoding Fibroblast-activating Protein, SDC4 encoding Syndecan-4, MUC1 encoding mucin-1, HMGA2 which is an independent marker for stromal activation,
15 ITGA5 encoding Integrin a5 subunit responsible for enhanced tumor-stroma interaction, and CD44, a membrane glycoprotein involved in ECM regulation of TGFβ pathway. While hypoxia increased expression of several genes regulating CAF-matrix interaction, those downregulating matrix degradation were also increased, including SERPINE1, PLAT, and PLAU, key inhibitors of the matrix metalloproteinases. Furthermore, SOX9, which is a key regulator of fibroblast activation was also increased in hypoxia, as well as PDGFC.
16,17 In addition to the genes associated with fibroblast activation, other key genes were involved in the glycolytic shift in metabolism, including PFKP encoding a member of the phosphofructosekinase family, as well as ENO1 and ENO2 encoding enolases, HK2 encoding hexokinase-2, and PGK1 encoding phosphoglyceratekinase. Hypoxia also resulted in reduced expression in FZD1 encoding Frizzled-1, SMURF2 which is a Smad specific ubiquitin ligase and is an inhibitor of fibrosis (
Fig. 1E).