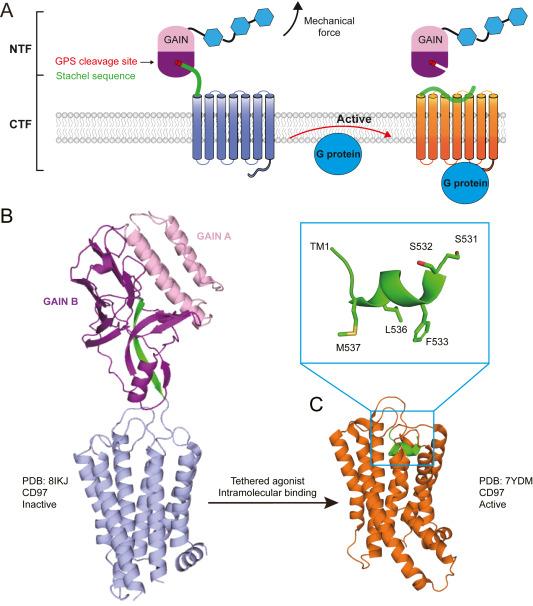
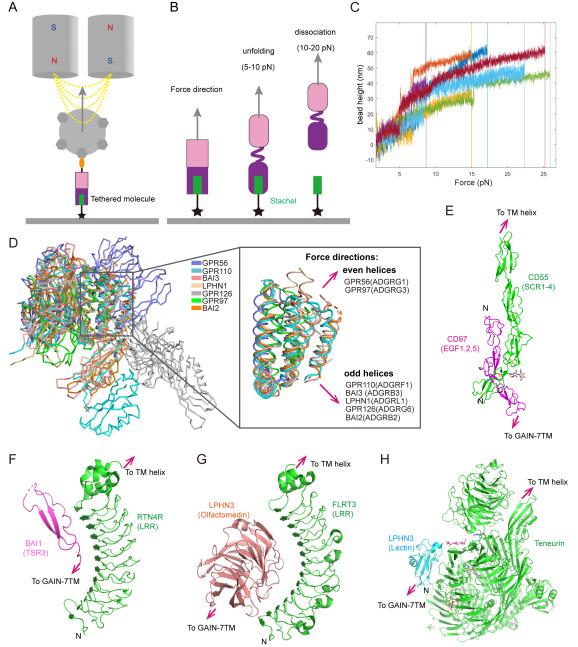
Using a single-molecule tweezer system, we and others have investigated the force response of GAIN domain-containing fragments of representative aGPCRs (
Fig. 3A).
36,37 Our findings demonstrate that when applying a force loading rate of 1.0 pN/s, the GAIN domains of ADGRG1 and ADGRL1 undergo structural unfolding within a force range of 5-10 pN, as evidenced by stepwise extensions of 10-30 nm. This step size indicated partial unfolding of the GAIN domain relative to the total extendable length of the loaded fragments (
Fig. 3B). Subsequent to these unfolding events, the
Stachel peptides dissociated from the GAIN domains within a force range of 10-20 pN in both receptors (
Fig. 3C). These data indicate that unfolding precedes
Stachel dissociation and that the unfolding likely occurs within the GAIN B subdomain, thereby facilitating
Stachel peptide dissociation by disrupting its interactions with surrounding residues in the GAIN B subdomain. These results are consistent with the notion that without partial unfolding,
Stachel peptide dissociation is less likely because of the anticipated catch-bond kinetics arising from the overall β-sheet structure of GAIN B. Thus, partial unfolding of the GAIN B subdomain may precisely disrupt the interaction network surrounding the
Stachel peptide, thereby facilitating its dissociation. Moreover, our single-molecule data suggested that dissociation between the GAIN A and B subdomains likely precedes the partial unfolding of GAIN B
36. Similarly, Zhong et al. found that the GAIN domain of ADGRL3/LPHN3 can be dissociated by physiologically relevant forces of 1-10 pN. Both studies support the mechanical activation hypothesis of aGPCRs, highlighting the sensitivity of the GAIN domain structure and its detachment to physiological forces. These results are consistent with a recent
in vivo study suggesting that NTF/CTF dissociation occurs
in vivo and that mechanical force is necessary for the activation of the neural latrophilin-type aGPCR Cirl from
Drosophila.
38