1. Introduction
2. Biomechanical and mechanobiological insights into biomaterial design
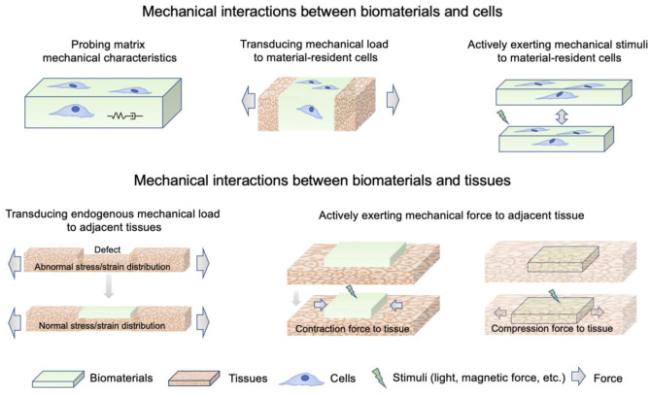
2.1. Mechanical interactions between biomaterials and tissues/cells
Fig. 1. Mechanisms involved in mechanical interactions between biomaterials and tissues/cells. |
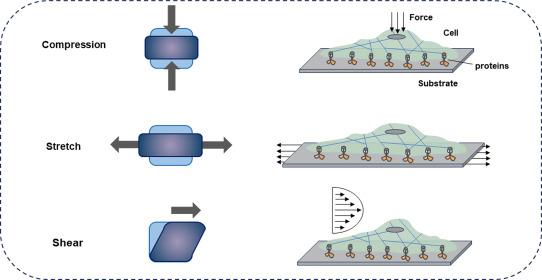
2.2. Transduction of mechanical signals at cellular level
Fig. 2. Typical modes of mechanical stimuli experienced by cells. External forces result in compressive, stretch (tensile), and shear stresses on cells.26 |
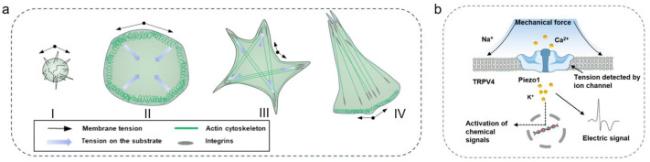
Fig. 3. Typical mechanisms involved in cell reception of mechanical signals. (a) Model of cell spreading and polarization. Phase 1 involves rounded up cells with weak cell-substrate attachment but higher membrane tension. In phase 2, the cells begin to spread by unfolding membrane reservoirs and increase the membrane tension. Stable adhesions start to mature during phase 3, global remodeling of the cytoskeleton occurs with formation of actin bundles and the membrane tension is now at its low resting level with a constant membrane area. In the final polarized phase, lamellipodia protrude on one side of the cell, whereas the other side of the cell shrinks and membrane tension increases.38,59,60 (b) Schematic diagram of ion channel activation by mechanical force. Schematic diagram of Piezo1 channel activation by mechanical force.61 |
Fig. 4. Representative schematic showing several typical mechanisms involved in cell mechanotransduction. |
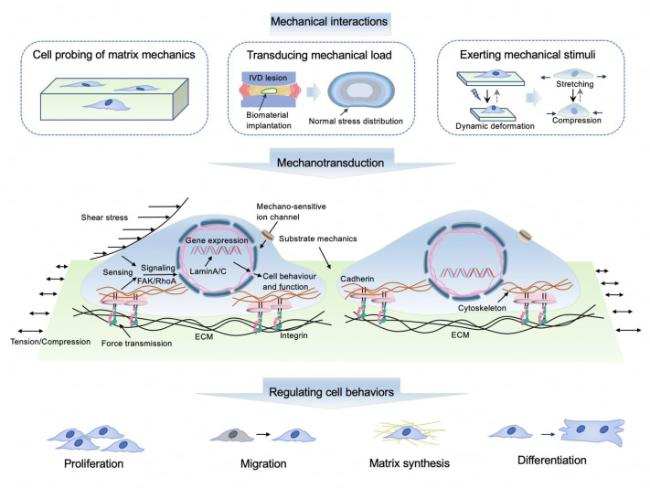
Fig. 5. Illustration of typical mechanotransduction mechanisms steering material- and tissue-resident cell behaviors. IVD: intervertebral disc. |
3. Effect of material mechanics on cellular and tissue responses
Table 1. Cellular processes that are potentially affected by matrix mechanical properties. |
| Stiffness | ||
|---|---|---|
| Matrix | Biological processes | Ref. |
| High-density collagen-I gels | Maintenance, proliferation, stratification, and survival of limbal epithelial stem cells | 105 |
| Polyethylene glycol hydrogel | Stemness and proliferative efficiency of skeletal muscle stem cell | 107 |
| Alginate-RGD hydrogels | Matrix remodelling of bone marrow mesenchymal stem cells | 106 |
| Hybrid gold nanoparticle-hyaluronic acid hydrogel | Electrical and contractile performance of human induced pluripotent stem cells | 108 |
| Trilayer extracellular matrix-based microribbon scaffold | Deposition of cartilage-like collagen and synthesis of sGAG of human mesenchymal stem cells | 109 |
| Beta-sheet rich silk nanofiber and amorphous silk nanofiber composite hydrogel | Chondrogenic-osteogenic gradient differentiation of bone mesenchymal stem cells | 189 |
| Proteolytically degradable alginate hydrogels | Migration and invasion of human mesenchymal stem cells | 111 |
| Alginate microgel | In vivo residence time of clonally derived mouse marrow stromal cells after intravenous injection | 112 |
| Four-armed poly(ethylene glycol) hydrogel | Organoids generation of human pluripotent stem cell | 116 |
| Gelatin-hydroxyphenylpropionic acid hydrogel | sGAG production and gene expression of chondrocyte | 103 |
| γ-PGA-SH/OHA-GMA hydrogel | Migration and infiltration of fibroblasts | 92 |
| Hyaluronic acid-based microrods | Reprograming of myofibroblast | 95 |
| Alginate hydrogel | Osteogenic differentiation of mesenchymal stem cells | 110 |
| Biphasic CAN-PAC hydrogel | Viability of cartilage cells and osteoblast cells | 96 |
| Viscoelasticity/dynamic network | ||
| Matrix | Biological processes | Ref. |
| Hyaluronate-alginate hybrid hydrogel | Chondrogenic differentiation of mouse chondrocytes (ATDC5 cells) | 190 |
| Benzimidazole-based catalyst enhanced hyaluronic acid hydrogel | Long-term survival and adhesion of human umbilical vein endothelial cells (HUVEC) | 148 |
| Supramolecular gelatin hydrogels | Spreading, tension of cytoskeletal and osteogenesis of human mesenchymal stem cells | 147 |
| PEG-gellan gum/PEGDA double network hydrogel | Proliferation, spreading and chondrogenic differentiation of bone mesenchymal stem cells | 157 |
| DEX-UPy dynamic hydrogel | Osteogenic differentiation of bone mesenchymal stem cells and chondrogenic expression of chondrocyte | 155 |
| Peptide supragel | Embedding and spheroid harvesting of cancer cells (MCF-7 and 4T1) | 161 |
| PPy-based dynamic conductive hydrogel | Differentiation towards astrocytes of neural stem cells | 164 |
| Gelatin methacryloyl -fibrinogen interpenetrating network hydrogel | 3D myoblast alignment and elongation of C2C12 cells | 165 |
| Collagen, alginate, and PEDOT:PSS biohybrid hydrogel | Physiological beating rate of human-induced pluripotent stem cell-derived cardiomyocytes | 166 |
| 3D hydrogel structure based on reversible receptor-ligand interaction between the glycopeptide antibiotic vancomycin and dipeptide D-Ala-D-Ala | Adhesion and morphology of L929 cells and HUVECs | 167 |
| Alginate hydrogel | Osteogenic differentiation and migration of hMSCs | 169 |
| GelMA hydrogels | Spreading, proliferation, osteogenesis and chondrogenesis of bone mesenchymal stem cells | 14 |
| PVA/Glycerol hydrogel | Vitality of nucleus pulposus cells | 11 |
| Strain-stiffening effect | ||
| Matrix | Biological processes | Ref. |
| Polyisocyanides gel | KCa3.1 channel expression of HepG2 cells | 130 |
| Viscosity | ||
| Matrix | Biological processes | Ref. |
| Polydimethylsiloxane-based substrate | Collective movement of epithelial cells | 181 |
| Hyaluronic acid hydrogel | Cell spreading of human bone marrow-derived mscs | 182 |
| Poly(ε-caprolactone)-based substrate | Spread area and formation of spheroids of NIH/3T3 fibroblast | 183 |
| Supported lipid bilayers | Differentiation of C2C12 mouse myoblasts | 184 |
| Gelatin solution | Adipogenic and osteogenic of human bone marrow-derived mesenchymal stem cells | 186 |
| ECM production and expression of collagen type II/aggrecan of chondrocytes | 185 | |
| Chondrogenic differentiation of human mesenchymal stem cells | 187 | |
| High-viscosity storage solution (EAS-1587) | Storage of red blood cell and inflammatory cytokines expression of human mesenchymal stem cells | 188 |
Fig. 6. Schematics of typical constitutive models for describing mechanical properties of biomaterials. (a) The stress-strain curve for a hyperelastic (Neo-Hookean) model. (b) The stress-strain curve of the polyisocyanopeptide hydrogel in a stress ramp. (c) Viscoelastic creep and stress relaxation under an instantaneous and constant applied stress (σ0) and strain (ε0).136 (d) Poroelastic behaviors of biopolymer networks under compression and shear tests.173 (e) Newton's law of viscosity. |
3.1. Time-independent mechanical properties
3.1.1. Stiffness/elasticity
3.1.2. Strain-stiffening behavior
3.2. Time-dependent mechanical behavior
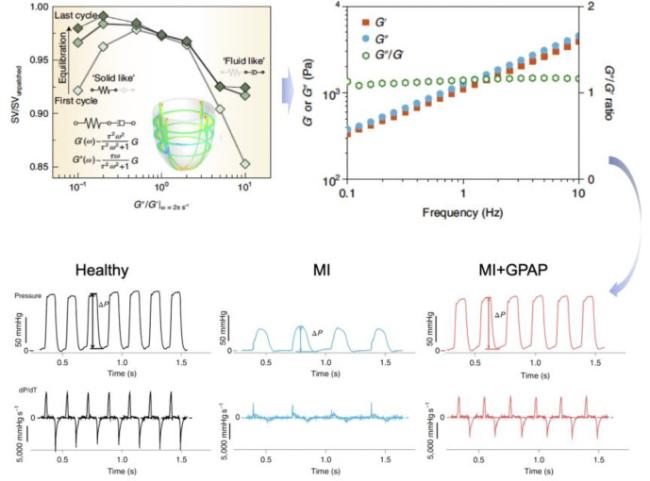
3.2.1. Viscoelasticity
3.2.2. Poroelasticity
3.2.3. Viscosity
3.3. Programming mechanical properties of biomaterials to control cell/tissue responses
4. Theoretical tools for deciphering mechanical interactions and designing optimal mechanics and geometry
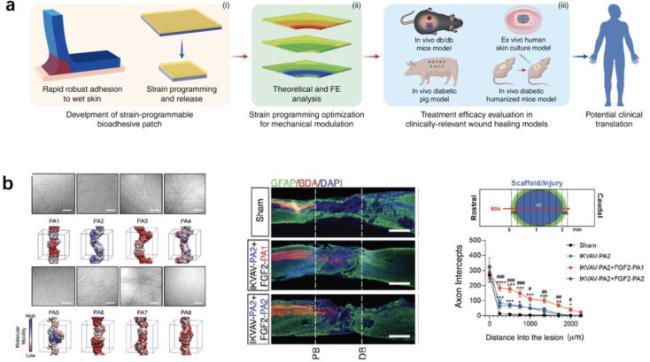
Fig. 7. Application of simulation approaches in the development of mechanobiomaterials. (a) With the aid of FEA, a strain-programmed patch was developed, which could mechanically contract diabetic wounds in a programmable manner to enhance the healing of diabetic wounds.217 (b) Guided by coarse-grained MD simulations, peptide amphiphiles (PAs) based assemblies with high internal dynamics was developed for spinal cord injury repair.163 |
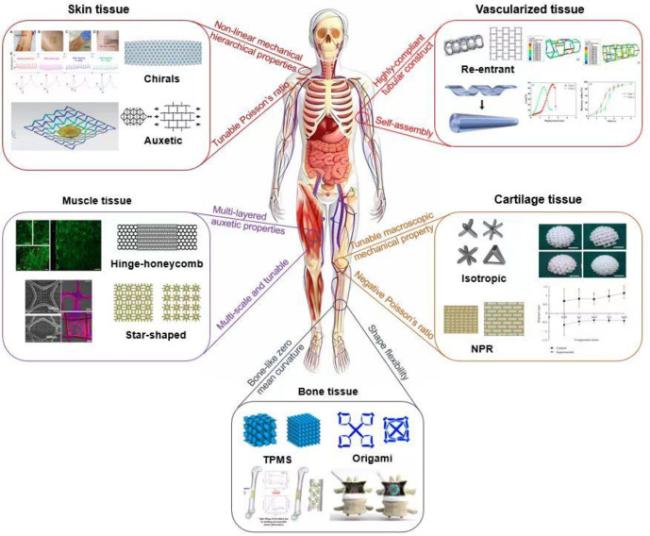
5. Rational design of mechanobiomaterials
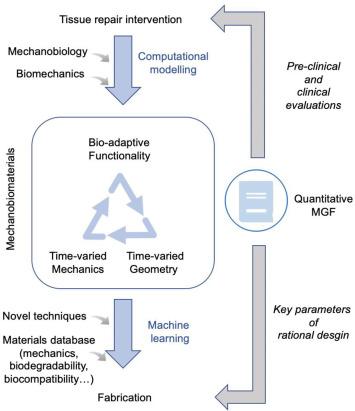
Fig. 8. A closed-loop workflow of designing mechanobiomaterials. |
5.1. Function-oriented optimization of mechanics and geometry
Fig. 9. Development of a mechanobiologically optimized epicardial patch using a closed-loop workflow of reverse design.10 |
5.2. Integration and synergy of material mechanics and biology
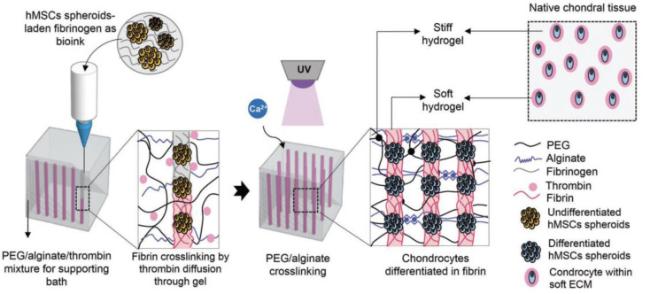
Fig. 10. Schematic of a 3D bioprinting approach for engineering cartilage-like mechanically hierarchical constructs with a hard biomaterial to mimic the macro-mechanical properties of native cartilage, and a soft biomaterial to create a chondrogenic microenvironment.222Spatiotemporal control of mechanical properties is also critical for the precisely reconstructing cell/tissue mechanical environment. An example is the development of a bilayer osteochondral scaffold with a 3D-printed Ti alloy subchondral bone compartment and a freeze-dried poly-lactic-co-glycolic acid (PLGA) reinforced collagen sponge as cartilage compartment was developed.223 The mechanical support provided by the 3D-printed Ti alloy layer facilitates long-term regeneration of cartilage by accelerating osteochondral formation and its integration with the adjacent host tissue. Recently, osteochondral scaffolds with spatially heterogeneous stiffness demonstrated the stiffness-specific induction of chondrogenic and osteogenic differentiation of stem cells for cartilage and bone regeneration, respectively.189 Most recently, the multidirectional stiffness design of artificial IVD demonstrated the ability to simulate the physiological kinematic behaviors of native IVD, bearing great potential in reconstructing the mechanical environment of adjacent tissues.224 |