1. Introduction
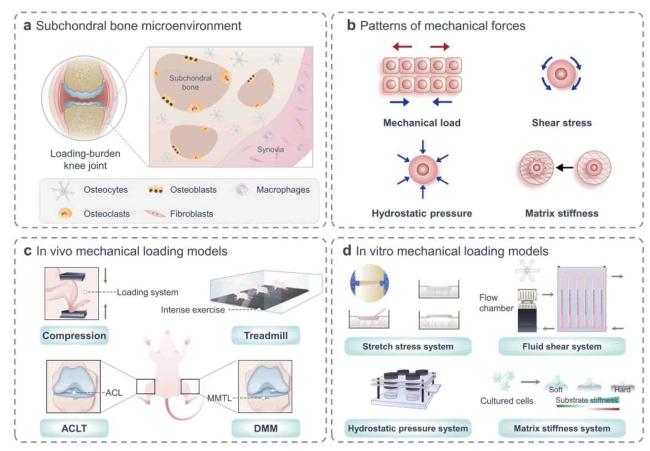
Fig. 1. Structural basis of mechanics in skeletal cells and models commonly used in mechanobiology. (a) Subchondral bone microenvironment contains osteocytes, osteoblasts, osteoclasts et al., all of which are sensitive to mechanical stimulation. (b) Mechanical stimuli applied to cells include mechanical load, shear stress, hydrostatic pressure, and matrix stiffness. (c) In vivo experiments: cycling mechanical compression is performed on the limb using a computational control machine; OA model is established by simulating the process of joint damage in running experiment; microsurgical techniques are used to perform ACLT and DMM models under direct visualization. (d) In vitro experiments: multi-channel cell stretch stress loading device can load uniaxial and biaxial stretches on cell and tissue cultures; multi-flow field cell fluid shear force loading culture and real-time observation/analysis system provide cells with various forms of fluid shear forces; continuous flow constant pressure for cell culture system uses hydrostatic water flowing into petri dishes to maintain target temperature, humidity, and carbon dioxide levels; isolated cells are seeded onto a polyacrylamide gel that mimicked different matrix stiffness. |
2. Mechanical forces on the joint
Table 1. Experimental conditions for in vivo mechanical loading models. |
| Animal model | Equipment | Body part | Mechanical force | Period | Brief summary | Refs. |
|---|---|---|---|---|---|---|
| Lewis rats | Non-Invasive Knee Injury device | Tibia | 5.4 N | 15-25 s followed by a single 3.26 ± 0.28 N/s ramp in compressive load | Non-invasive knee injury models produce consistent ACL breakage and lead to conventional OA pathology without directly damaging other tissues. | 17 |
| Mice | Loading system used for cyclic compression | Lower leg | 7-10 N | 1200 cycles per day, 5 days per week for multiple weeks | Joint injury can be induced by cyclic compression load or acute overload to induce ACL rupture. | 18 |
| Wistar rats | DMM surgery | Knee | 21 m/min intense treadmill exercise | 30 min/day, 5 days/week for 4 weeks | Exercise modulates the development of post-traumatic OA in a dose-dependent manner during DMM surgery. | 20 |
3. Perception of subchondral bone cells to mechanical force patterns
Table 2. Experimental conditions for in vitro mechanical loading models. |
| Cell type | Mechanical stimulation | Duration | Response | Refs. |
|---|---|---|---|---|
| MC3T3-E1 osteoblast | Fluid shear stress | 0.70 Pa, 0.70 ± 0.31 Pa, 0.70 ± 0.70 Pa, 5 or 9 Hz | NO production is a parameter of osteoblast activation linearly dependent on the rate of fluid shear stress. | 27 |
| Osteocyte | Dynamic substrate strain; fluid flow | 0.1-10% 1 Hz; 2 N/m2 1 Hz | Fluid flow plays a greater role than matrix deformation in bone cell mechanical transduction associated with bone adaptation to conventional load. | 28 |
| Osteocyte | Fluid pressure | 8 dyn/cm2 | During cyclic loading, tracer transmission increases with increasing load amplitude and permeability, and decreases with increasing loading frequency. | 31 |
| Normal human osteoblast | Shear stress | 20 dyn/cm2 | Fluid flow shear stress induces the proliferation and differentiation of osteoblasts through various signaling pathways. | 34 |
| MG63 osteoblast-like cell | Shear stress | 0, 1, 5, 14, and 30 dyn/cm2 | Decrease in shear force-mediated osteoblast differentiation may be due to the increased production of PGE2. | 35 |
| Osteoclast precursor RAW264.7 cell | Low-fluid shear stress | 0.1-0.7 Pa | Osteoclast precursors sense fluid shear stress gradients and tend to actively migrate to regions with low fluid shear stress, which are regulated by Ca2+ signaling pathways. | 42 |
| Co-cultured osteoclast | Mechanical stress | Cyclic loading at 1 Hz | Mechanical stress-induced TGF-β1 overexpression of osteoclast is the cause of OA chondrocyte apoptosis and cartilage degeneration. | 46 |
| Bone marrow-derived macrophage and RAW264.7 monocyte | Different stiffness degrees | Substrates 1:5 (E = ∼4.05 MPa) and 1:45 (E = ∼0.1 MPa) | Harder PDMS substrates accelerate osteoclast differentiation. | 114 |
| Osteoclast of rabbits | Intermittent tensile strain | Magnitude 1730 μϵ | Mature osteoclasts independently sense mechanical stimulation and upregulate bone resorption activity. | 115 |
3.1. Osteocytes
3.2. Osteoblast lineage cells
3.3. Osteoclast lineage cells
4. Mechano-signaling in subchondral bone cells
Table 3. Mechanosensing structures and mechanotransduction signaling pathways of the subchondral bone microenvironment. |
| Cell type | Mechanical stimulation | Mechanical signaling | Major outcomes | Refs. |
|---|---|---|---|---|
| Osteocyte | Fluid flow stress | Integrin αvβ3 | Focal mechanical stimulation of osteocytes occurs along processes but not cell body and αvβ3 integrin plays an essential role in osteocyte activation. | 55 |
| Fluid-flow induced forces | Matrix-dependent adhesion | Direct interstitial junctions between MLO-Y2 cells in a connected osteocyte network are coupled with extracellular purinergic P4 receptor signaling in response to mechanical signals. | 56 | |
| ECM stiffness | YAP/TAZ | YAP/TAZ activity is induced by ECM hardness and cell diffusion in stressed fiber and cytoskeletal tension. | 58 | |
| Dynamic fluid flow | Primary cilia | Primary cilia in bone transfer fluid to cellular responses in osteocytes independent of Ca2+ fluxes and stretch activated ion channels. | 61 | |
| Static and fluid flow | Piezo1 | Over-expression of Piezo1 in MLO-Y4 cells increases the expression of Ptgs2 and Tnfrsf11 b and enhances the response of the fluid shear stress. | 90 | |
| Mesenchymal stem cell | Hydrostatic pressure | Piezo1 | 0.01 MPa hydrostatic pressure is the most suitable to induce MSCs differentiation into osteoblast lineage cells and induce osteoblast differentiation. | 88 |
| Osteoblast | Shear stress | TRPM7 | TRPM7 is mechanically sensitive to shear forces of 1.2 Pa, much lower than the recently reported pressure loads of 98 Pa, and mediates different mechanical transduction pathways. | 92 |
| Hypo-osmotic stress | TRP channel | Hypo-osmotic stress induces Ca2+ influx through TRPM3 and TRPV4 to regulate RANKL and NFATc1 expression. | 96 | |
| Tensile stress | Wnt/β-catenin | Tensile stress can promote the expression of β-catenin protein in preosteoblasts at the initial stage of the experiment, but reduce at 12 h and 40 h after the end of periodic loading. | 100 | |
| Strain/stretching | Runx2 | Runx2 affects osteoblast function and mediates mechanical transduction by binding to osteoblast-specific cis-acting element 2. | 108 | |
| Osteoclast | Substrate stiffness | Cytoskeletal arrangement | Extracellular substrate hardness is an essential determinant of osteoclast differentiation and function, and stiffer stiffness accelerates osteoclast differentiation. | 114 |
| Mechanical stretching | SA-cat channel | Mature osteoclasts respond to mechanical stretching through mechanisms involving the SA-cat channel and thus up-regulate bone resorption activity. | 115 | |
| Fluid flow | STIM1 and TRPV4 | Intracellular Ca2+ oscillations in osteoclasts mechanically stimulated by fluid shear force induce STIM1 to be highly expressed in early osteoclasts and TRPV4 to be highly expressed in late osteoclasts. | 116 | |
| Substrate rigidity | Podosomes | By detecting the stiffness and topography of the matrix, the podosomes mechanically sense both intracellular and extracellular signals generated downstream. | 119 |
4.1. Osteocytes’ perception and response to mechanical signals
4.1.1. Cytoskeleton and cilia
4.1.2. Ion channels
4.1.3. Connexin 43
4.1.4. Wnt pathway
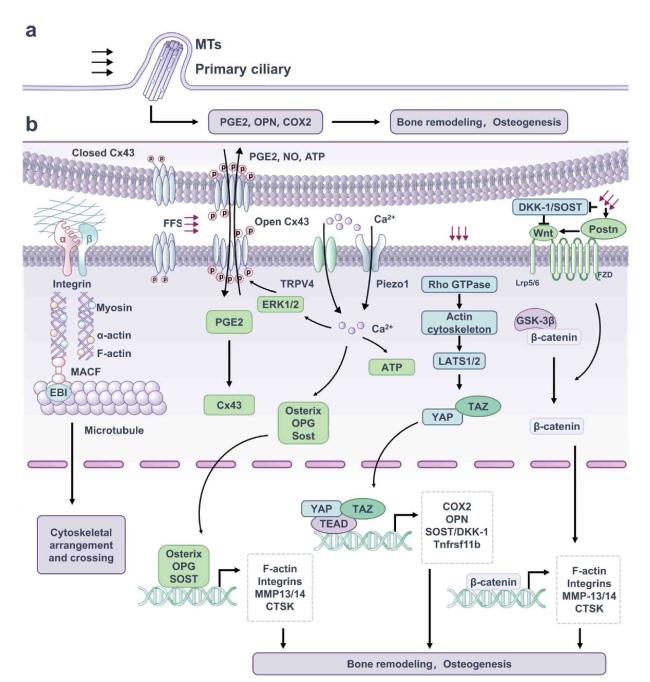
Fig. 2. Mechanosensitive structure and signaling pathway of osteocytes. (a) Primary cilium is a special cell protrusion structure consisting of 9 doublet MTs in the shape of a “9 + 0” pattern, which transmit mechanical signals via the participation of ciliate protein. (b) Integrin adhesion complexes connect ECM and F-actin cytoskeleton and enhance activation of downstream pathways. Upon subjected to mechanical stimulation, Cx43 protein is phosphorylated, the linker is turned on, causing the increase of PGE2 and maintaining bone remodeling. The earliest event in osteocyte mechanical transduction is the increase in intracellular Ca2+ concentration. This process is mobilized by which mechanical stimulation increases the osteocyte membrane tension, further inducing the opening of Piezo1, TRPV4 channels, Ca2+ influx, and further promoting osteogenesis and maintaining bone homeostasis. Mechanical stimulations modulate Rho GTPase activity, leading to actin cytoskeletal remodeling, which controls the nuclear-cytoplasmic shuttle and transcriptional activity of YAP/TAZ. Typical Wnt/β-catenin pathway is activated by Wnt ligand binding to a co-receptor complex consisting of Lrp5 or Lrp6 and FZD, and GSK-3β is phosphorylated to release the captured β-catenin. As a result, free β-catenin is transferred to the nucleus to induce downstream gene transcription, which promotes bone formation. |
4.2. Mechanotransduction in osteoblast lineage cells
4.2.1. Mechanosensitive ion channels
4.2.2. Wnt/β-catenin pathway
4.2.3. Other mechanical cascades
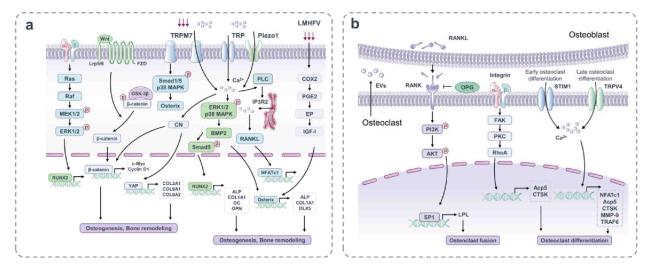
Fig. 3. Mechanosensitive structures and signaling pathways of osteoblasts and osteoclasts. (a) Membrane binding receptors such as integrins, TRPM7, Piezo1 and so on are stimulated by mechanical forces to induce multiple transcription factors that regulate osteoblast differentiation and formation. Mechanical stimulation activates the typical Wnt/β-catenin signaling pathway and affects osteogenesis and bone remodeling. LMHFV activates the COX2-PGE2-EP pathway to stimulate osteoblast differentiation. (b) Integrin αvβ3 is activated by mechanical stimulation, followed by a biochemical signaling cascade of FAK, PKC and RhoA, which is associated with osteoclast differentiation. In addition, there is a close relationship between osteoclasts and osteoblasts. After RANKL from mechanical stimulated osteoblasts, the expression of LPL in preosteoclasts is increased, which is regulated by PI3K/AKT/SP1 pathway and promotes the formation of mature osteoclasts. STIM1 and TRPV4 act as mechanical transduction channels of osteoclasts in the early and late stages of differentiation. |