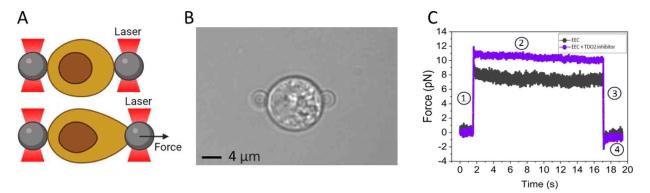
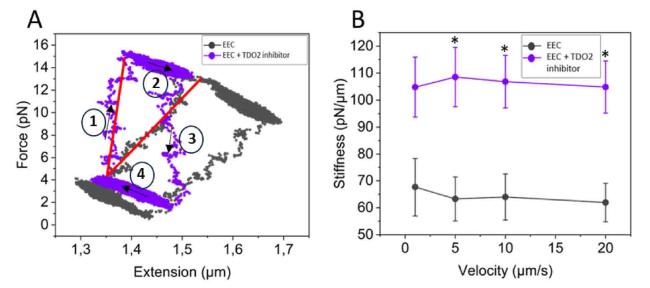
We present the first mechanical characterization of serotonin-producing EECs, exhibiting stiffness values ranging from 60 to 70 pN/μm at stretching velocities ranging from 1 to 20 μm/s. These stiffness values are a magnitude higher than the stiffness values we previously observed for red blood cells,
24 and slightly lower than those for primary human monocytes.
25 In addition, we showed that upon TDO2 inhibition, EECs show significant stiffening. TDO2 plays a pivotal role in the catabolic pathway of tryptophan (an essential building block for proteins and precursor for biologically active compounds such as the neurotransmitter serotonin), where it catalyzes the conversion of tryptophan into kynurenine.
26 This enzymatic process is the initial and rate-limiting step in the kynurenine pathway. Quinolinic acid (QUIN) is an endogenous downstream metabolite of the kynurenine pathway.
26 TOD2 inhibition therefore leads to a reduction in QUIN production. QUIN has been shown to disrupt cytoskeletal filaments through hyperphosphorylation.
27 Lower levels of QUIN might therefore explain the stiffening observed upon treatment with 680C91. However, TDO2 has also been shown to promote cancer progression by mediating tryptophan deprivation and the production of metabolites along the kynurenine pathway. The depletion of tryptophan has been shown to facilitate tumor immune escape by inhibiting the function of T cells.
28 Furthermore, the metabolites produced in this pathway can provide a source of energy for cancer cells, aiding their growth. Indeed, TDO2 has been shown to promote proliferation, migration, and invasion of various cancer cell types,
29,30,31,32,33 and especially serotonin-producing neuroendocrine tumors express TDO.
34 TDO2 inhibition and the associated cell stiffening might therefore offer a potential avenue for reducing gut cancer cell invasiveness and preventing metastasis.