Macrophages can modify their morphology in response to alterations in substrate stiffness (
Fig. 1). This phenomenon was also observed when macrophages were cultured on soft hydrogels, resulting in a more elongated form than macrophages cultured on rigid hydrogels, as indicated by a reduced minor axis.
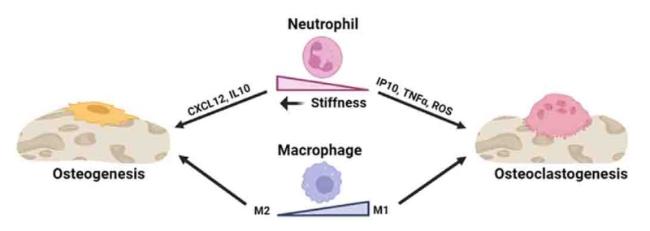
35 Moreover, macrophages can promptly adapt to their phenotype and polarization state in response to localized stimuli, including physical signals. One study revealed that hydrogels with a stiffness of 29.2 kPa effectively induced M1 macrophage polarization, as evidenced by elevated secretion of TNF-α and IL-6 and reduced secretion of TGF-β and C-C motif ligand 17 following a 3-day culture period.
35 Additionally, previous studies have consistently demonstrated that decreased substrate stiffness promotes M2-like macrophage activation, consistent with the findings of other studies.
57,58 Moreover, previous studies examined the influence of matrix stiffness on the osteogenic capacity of BMSCs, specifically exploring the role of macrophages in co-cultures to modify the effects of matrix stiffness on BMSCs.
59 In contrast, macrophages residing within high-stiffness gels exhibited an increased inclination toward polarization toward the proinflammatory M1 phenotype. By employing either conditioned medium-based incubation or transwell-based co-culture, macrophages encapsulated within a low-stiffness matrix were shown to benefit the osteogenesis of co-cultured BMSCs. Conversely, macrophages residing within high-stiffness gels adversely influence the osteogenic differentiation of cells.
59 However, other studies have reported contradictory results. After confirming the biocompatibility of the hydrogels, experiments were conducted using bone marrow-derived macrophages (BMMs) from mice incubated with different hydrogels. When exposed to simulated low substrate stiffness (2.55 ± 0.32 kPa), the BMMs exhibited increased expression of CD86 on their cell surface, increased production of ROS within the cells, and increased secretion of IL-1β and TNF-α in the supernatant. In contrast, BMMs exhibited heightened CD206 expression, decreased ROS production, and enhanced secretion of IL-4 and TGF-β when exposed to medium stiffness (34.88 ± 4.22 kPa).
34 The disparate outcomes observed can be attributed to using different substrate stiffness levels in the investigations.