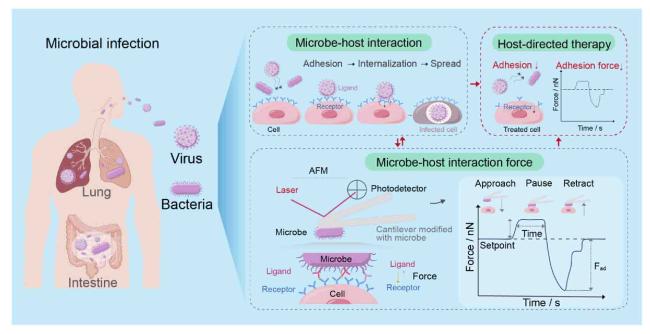
Recently, we have employed the FluidFM-based SCFS to quantify interfacial adhesion forces between pathogenic bacteria,
e.g.,
S. aureus and
Escherichia coli (
E. coli) and host cells,
e.g., IEC-6 cells (rat small intestinal epithelial cell line-6) and HaCat cells (human keratinocyte cell line) (
Fig. 1), and therefore developed an
in vitro model to quantitatively study bacterial-host cell interactions mediated by mechanical microenvironments of extracellular matrices.
7 Based upon a well-developed microcontact printing approach, we first established a series of host cell monolayers with different geometric shapes and sizes on collagen-coated polyacrylamide (PAAm) substrates whose rigidities were 10.14 kPa (soft), 32.29 kPa (medium) and 93.46 kPa (stiff), respectively. Subsequently, we explored the spatiotemporal dynamics of interfacial interactions between the geometrically confined host cell monolayers and the pathogenic bacteria expressing green fluorescent protein (GFP).
7 Surprisingly, most bacteria always adhered near the edges of the geometrically micropatterned host cell monolayers, showing strong spatial heterogeneity during the bacterial-host interactions. Further, we revealed that bacterial (
S. aureus) adhesion forces increased linearly along the radial direction of a circular IEC-6 cell monolayer (200 μm in diameter), approximately. These facts demonstrate that spatially geometric constraints on host cell monolayers intrinsically play a key role in modulating bacterial-host interactions. Also, our experimental results showed that the interfacial adhesion forces were directly related to the underlying substrate stiffness. Specifically, the peak adhesion force near the outer edges of the circular cell monolayers cultured on the soft substrates was ∼40 nN while it increased to ∼75 nN when the cell monolayers were cultured on the stiff substrates.
7 These findings imply that the interfacial adhesion is susceptible to mechanical aspects of extracellular matrices as well.