1. Introduction
2. General principles of current immunodiagnostic tests
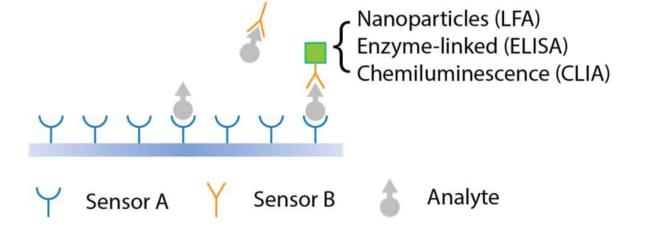
Fig. 1. Schematic illustration of sandwich-type components used in immunodiagnostic tests. |
3. Commonly applied immunodiagnostic technologies
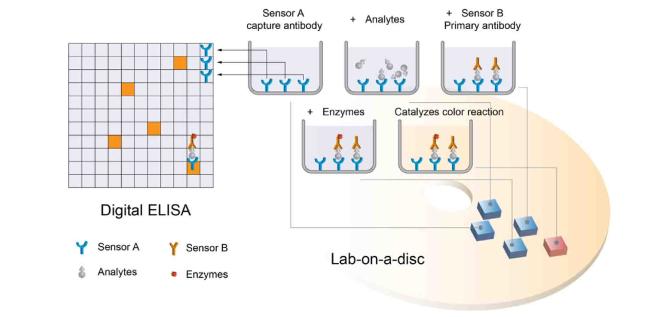
Fig. 2. Illustrates the crucial steps in a sandwich ELISA. Following the incubation of analyte molecules with the sensor A-coated surface, it is essential to eliminate the free analyte molecules before introducing the sensor B solution. Likewise, the removal of free sensor B is necessary before adding the enzyme solution. The integration of Digit ELISA enables the achievement of single-molecule sensitivity, while lab-on-disc platforms extend its application to point-of-care testing (POCT).4,16 |
Fig. 3. Schematic of an LFA depicting its general working principle and the sandwich-type components. The numbers within the figure highlight the strategies derived from each component, aimed at addressing the limitations of current LFAs: (1) implementation of sample preconcentration,34,35,36 (2) improvement of receptor immobilization platforms to control the flow rate,37,38,39 (3) integration of novel nanomaterials as signal transducers40,41,42 and (4) innovation in new readers for signal amplification.43,44,45 |
4. Common challenges of immunodiagnostic technologies
5. The single-particle mechanical selection (SPMS) technology
Fig. 4. Schematic of general principle of SPMS. (A) Microbeads situated near the bottom surface generate hotspots conducive to the formation of specific sensor A/analyte/sensor B complexes. (B) Enhanced specificity is attained by utilizing force-dependent dissociation of microbeads, capitalizing on the extended lifespan of microbeads specifically bound compared to the majority of nonspecifically attached microbeads. Following the precise application of controlled force over a calibrated period, the predominant population of retained microbeads is affixed through the specific sensor A/analyte/sensor B immunocomplex. The density of these retained microbeads serves as a direct signal readout. (C) Images of the retained microbeads, automatically counted with an algorithm. (D-E) Images of retained microbeads at different concentrations of CR3022, an RBD IgG, spiked in RIPA buffer (D) or in diluted blood samples at a fixed initial CR3022 concentration of 20 nM (E). Reprinted (adapted) with permission from Ref. 30. |
5.1. Mechanically enhanced specificity
5.2. Convenient readout signals
5.3. Rapid formation of specific sensor A/analyte/sensor B immunocomplex
5.4. Decreased impact from the Hook Effect
6. Applications of the SPMS technology
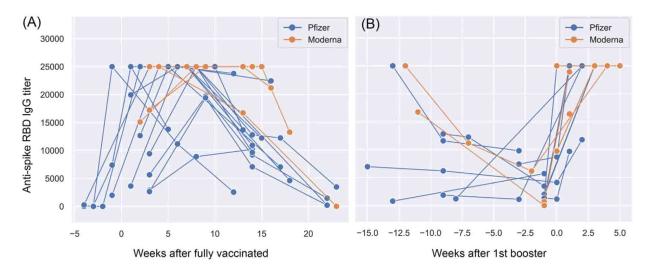
Fig. 5. Quantitative analysis of anti-RBD (Wuhan-Hu-1) antibodies level for infection and vaccine naïve individuals. (A) The titers of anti-RBD IgGs over time, measured in weeks after receiving the second dose on day zero of either the Pfizer-BioNTech (blue) or Moderna (organe) mRNA vaccine. (B) The impact of the first mRNA vaccine booster on the titer of anti-RBD antibodies. Reprinted with permission from Ref. 30. |
Fig. 6. Exploring diverse applications of SPMS: (A-B) The retained microbead density as a function of SARS-CoV-2 N-Ag (Omicron BA.2, Acrobiosystems) (A) and hCG antigen (BioResearch) (B) concentrations. (C-D) Detecting plasmid-expressed GPR56, an adhesion GPCR presented in HFF (C) and 293 T (D) cell lines, in 5X diluted cell lysates. In all these illustrative cases, we employed 30-min SPMS assays. |