Colon cancer is one of the most common malignant tumors and ranks second in terms of cancer-related deaths worldwide.
1,2 However, distant metastasis is considered the primary cause of clinical treatment failure in patients with colon cancer,
3,4,5 with an overall 5-year survival rate accounting for only 13.3%.
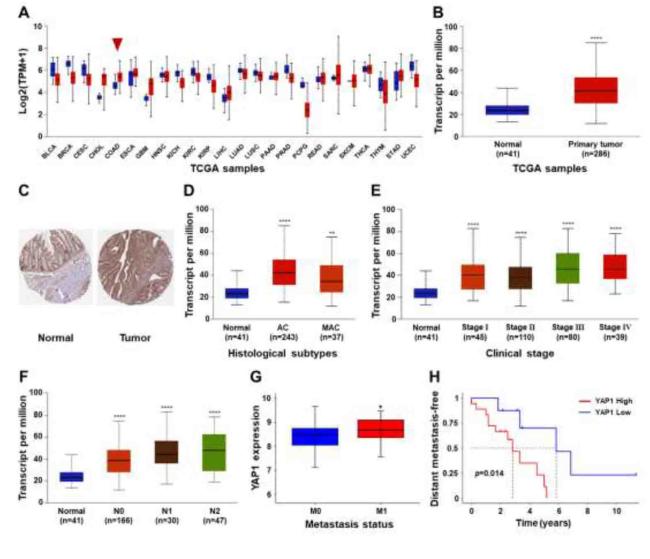
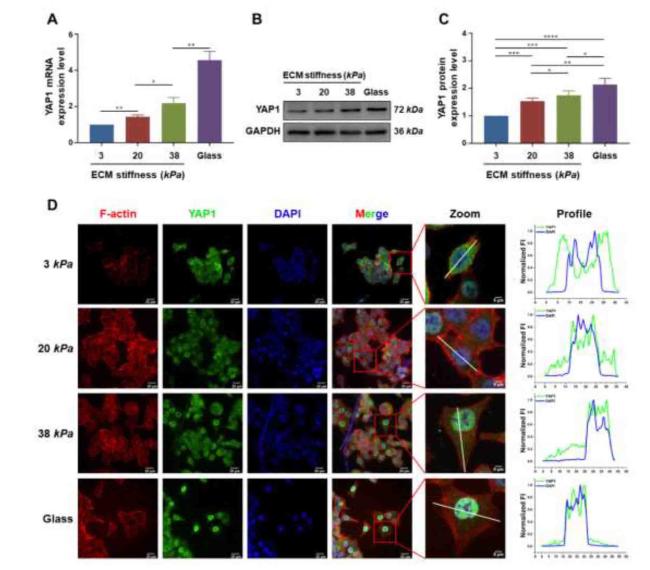
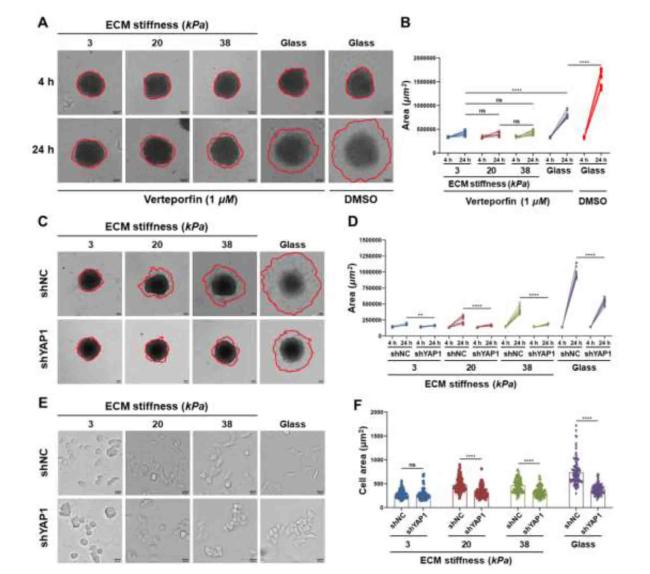
6 Our bioinformatics analyses found that the higher expression levels of yes associated protein 1 (YAP1), which is a core transcriptional coactivator and transducer of mechanical stimuli in patients with colon cancer, were associated with their metastasis and poor prognoses. Physical and chemical factors in the solid tumor microenvironment are key to regulating the occurrence and development of cancer cells.
7,8 An increasing number of researchers have focused on the mechanochemical coupling mechanism of the effects of increased extracellular matrix (ECM) stiffness, blood flow/interstitial flow shear stress, and static pressure on tumor cell proliferation and metastasis.
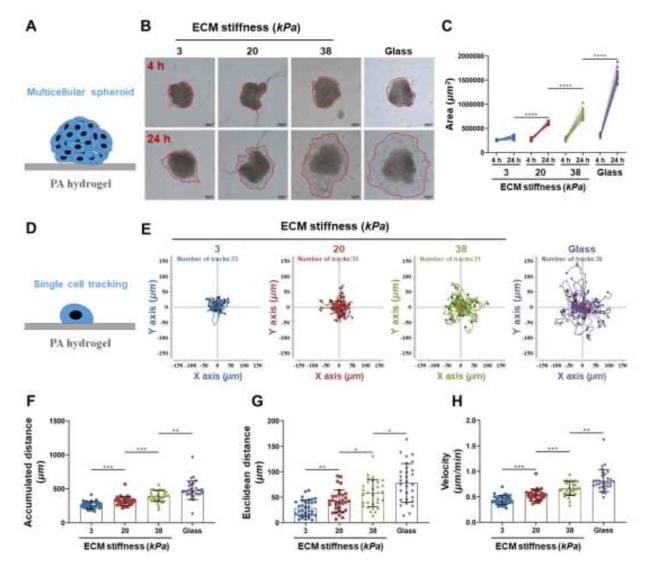
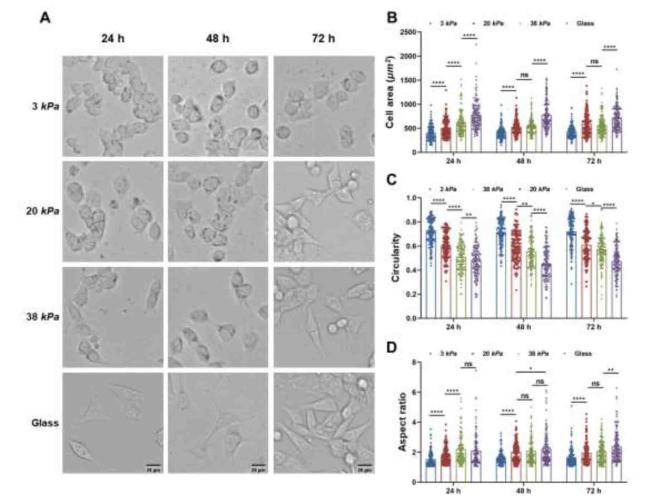
9,10 ECM stiffness plays an important role in the regulation of cancer cell migration and invasion.
11,12 Cells can sense and respond to various extracellular mechanical stimuli to ensure proper physiological functions, such as adhesion, migration, invasion, and progression of various diseases, including cancer.
13,14 Extracellular mechanical signals are transmitted by activating intracellular signaling pathways via mechanical sensors in the cell plasma membrane.
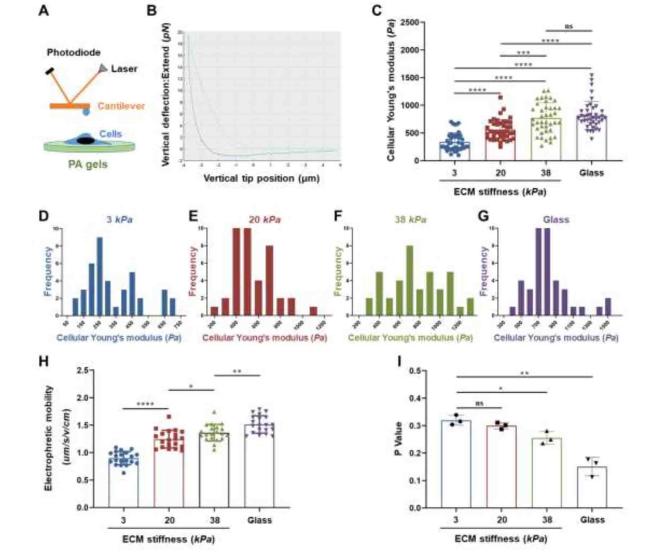
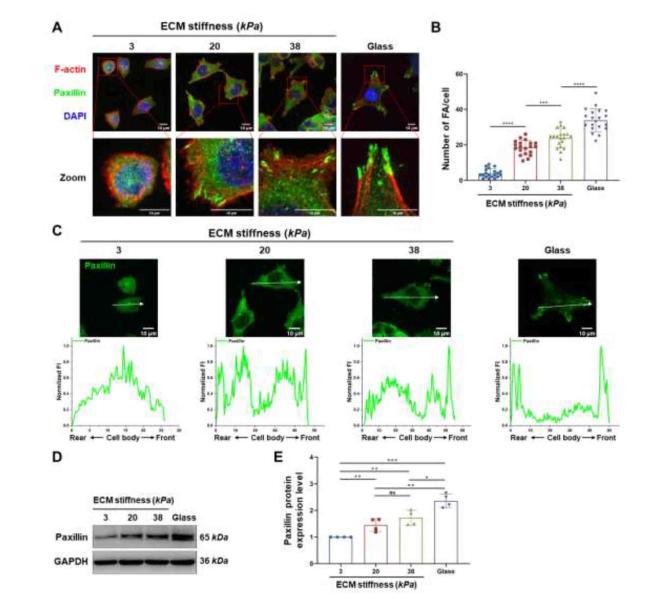
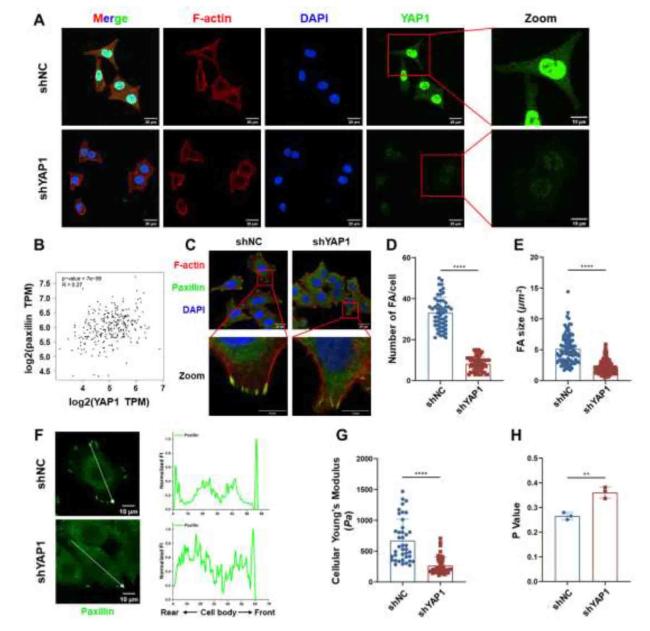
15,16 The force applied to ECM-integrin adhesion can activate focal adhesion kinase (FAK) and promote the formation of focal adhesions (FAs), thereby recruiting adhesion-related proteins (e.g., vinculin, talin, and paxillin) to trigger downstream signaling cascades.
17 FAs are dynamic multi-protein complexes that participate in ECM adhesion and play an important role in translating ECM stiffness signals into intracellular chemical responses. The dynamic cycle of FAs assembly and disassembly at the edge of a migrating cell provides a directional force for movement; FAs dysfunction is an important step in tumor cell invasion and metastasis.
18 Therefore, it can be hypothesized that colon cancer cells (CCCs) sense and respond to changes in ECM stiffness via YAP1 and FAs, leading to invasion and metastasis.