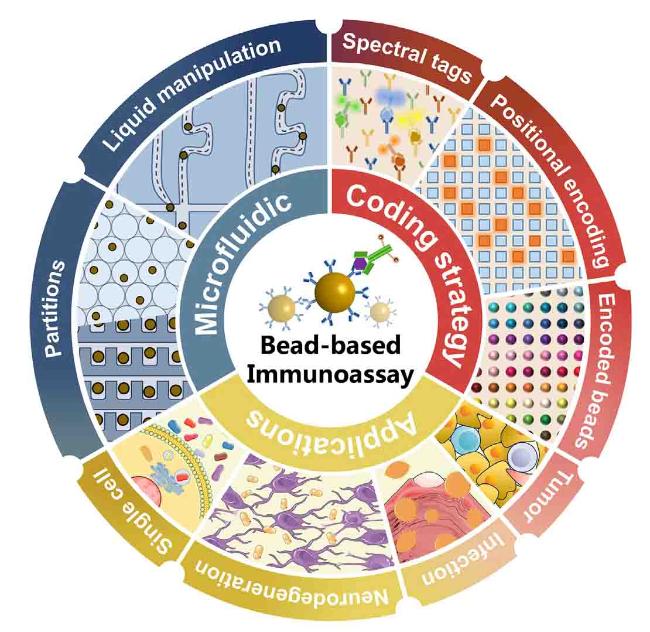
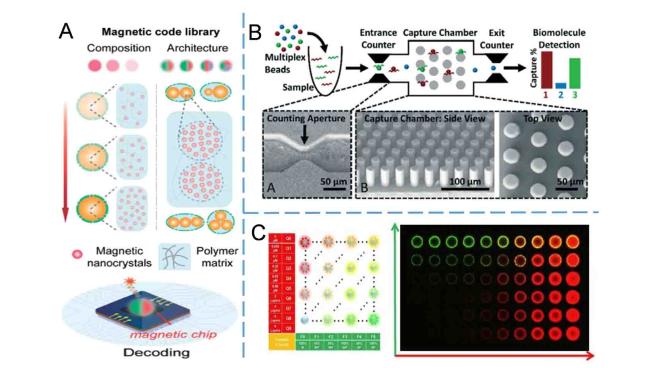
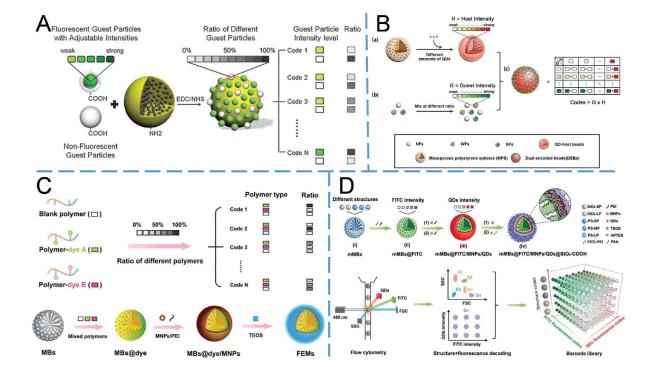
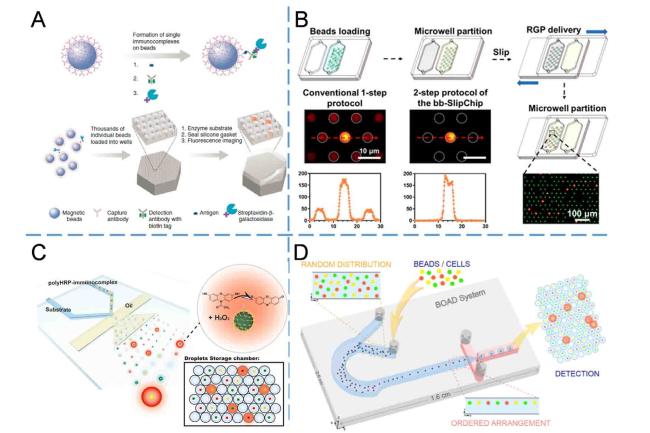
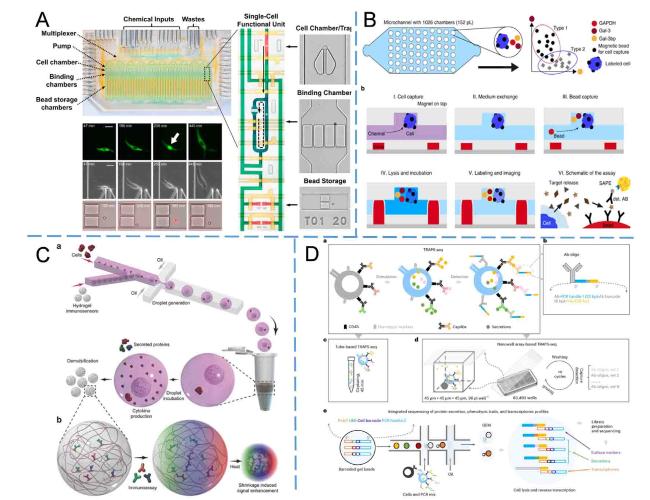
Electrochemical detection has been extensively employed in microfluidic devices
75 for highly sensitive immunoassay in POCT due to its straightforward detection apparatus, easy integration, and potential for multiplexed detection. Beads are commonly employed as carriers for enzyme labeling in electrochemical detection, while multiplex detection is typically accomplished by positional encoding within the microfluidic channels (
Fig. 6C). Rusling's research group employed 1 μm superparamagnetic beads conjugated with antibodies and HRP labels for off-line target capture and reduction of nonspecific binding.
76 Subsequently, the immune complex was introduced and immobilized by corresponding antibodies labeled on distinct electrodes within the detection channel. The quantification of PSA and IL-6 proteins with LOD of sub-pg/mL was achieved by monitoring the amperometric signals generated during the catalytic process of HRP within an 8-electrode screen-printed carbon array microfluidic device. By augmenting the quantity of antibodies and enzymes in the beads, the detection sensitivity of IL-6, IL-8, vascular endothelial growth factor (VEGF), and VEGF-C was further enhanced to 5-50 fg/mL.
77 Additionally, the integration of magnetic bead-based immune reaction and washing step in the microfluidic chip was achieved by incorporating a magnetic capture module and a mixing module at the bottom of the microfluidic chip.
78 As a result, simultaneous detection of IL-6 and IL-8 with LODs ranging from 5 to 7 fg/mL was accomplished within 30 min. Similarly, utilizing the aforementioned methodology and platform, simultaneous detection of four biomarkers (TNF-α, IL-6, IL-1β, and C-reactive protein (CRP)) in 5 μL serum samples was conducted with LODs ranging from 10 to 40 fg/mL.
79 Otieno et al.
80 utilized this platform for the multiplex detection of intact parathyroid hormone-related peptide (PTHrP) 1-173, along with circulating N-terminal and C-terminal peptide fragments, achieving a LOD of 150 aM. Moreover, they applied this method in diagnosing clinical cancer patients, thereby demonstrating its potential application in POCT. In terms of the detection throughput, this research group replaced the original 8 sensors with eight modules consisting of 32 sensors each in a miniaturized electrochemical module, enabling simultaneous analysis of 256 measurements within 1 h.
81 To explore the limits of detection sensitivity in this system and further enhance reaction kinetics, Rusling's group
21 subsequently replaced the magnetic bead-based enzyme signal amplification unit with a smaller polyHRP with higher enzyme load per unit. Consequently, improved LODs of sub-fg/mL were achieved for four proteins using gold nanoparticle coated sensors.