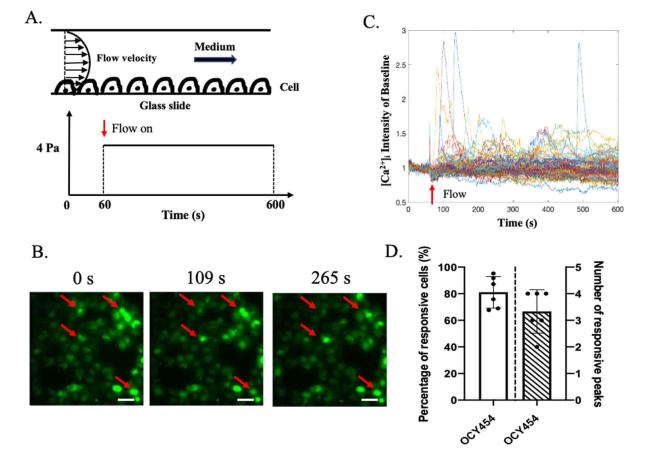
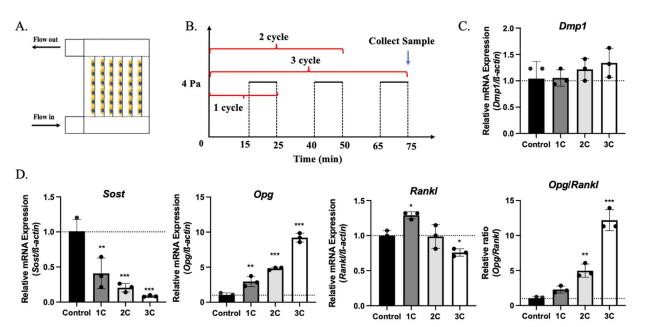
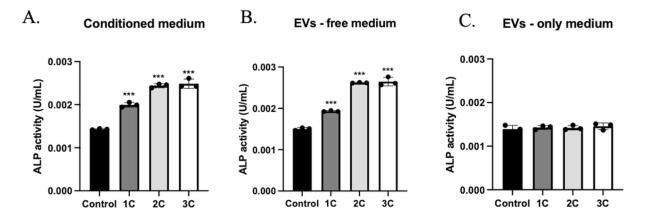
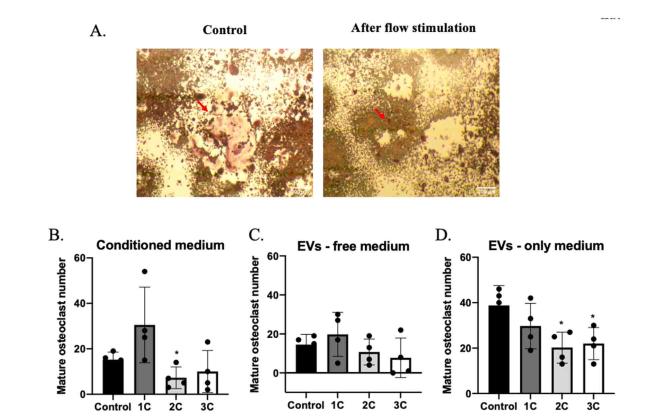
To study the response of OCY454 cells to mechanical loading in different loading durations, one-cycle burst (1C), two-cycle bursts (2C), and three-cycle bursts (3C) stimulation patterns have been used to apply SFF on OCY454 cells (
Fig. 2A and
B). After 75 min of processing, cells, and supernatant from all the groups were collected immediately. From qPCR results, we found that
Sost gene expressions have been downregulated, and
Opg gene expression has been upregulated in stimulated OCY454 cells compared with the non-stimulated control group (
Fig. 2D). Moreover, this effect was does-dependent on the loading duration. Unlike
Sost gene expression, which decreased in all the stimulation groups,
Rankl gene expression has been upregulated at the beginning and downregulated afterward in the 2C and 3C groups. The
Opg/Rankl ratio has increased significantly after stimulation. Even though
Dmp1, one of the mature osteocyte marker genes,
23 mRNA expression level has increased trend, they did not have statistical significance in all the groups (
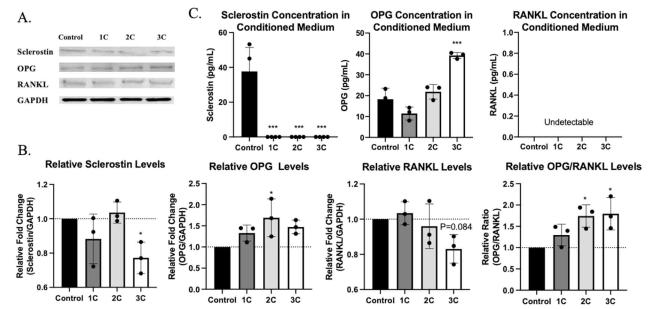
Fig. 2C). Similar to mRNA expression results, sclerostin protein levels in cells were decreased in flow stimulated 3C group and the concentration of sclerostin in the medium was reduced in flow stimulated group (undetectable) compared with the control group (
Fig. 3A,
B, and
C). OPG levels in cells and concentration in the conditioned medium were upregulated in response to mechanical stimulation (
Fig. 3A,
B, and
C). OPG/RANKL ratio in cells has significantly elevated in response to 2C and 3C fluid flow stimulation (
Fig. 3B). However, no RANKL has been detected in the conditioned medium (
Fig. 3C). These results suggest that sclerostin and OPG/RANKL ratio have been regulated in response to SFF stimulation in OCY454, and this regulation was load-duration dependent.