1. Introduction
2. Materials and methods
2.1. Materials
2.2. Custom-made parallel flow chamber
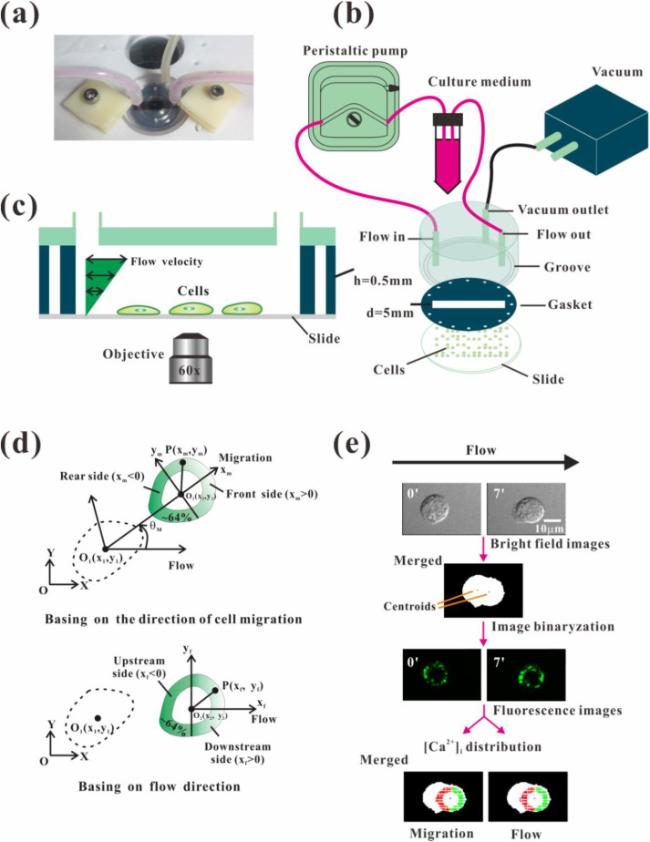
Fig. 1. Flow chamber and image processing. (a) Custom-made parallel flow chamber. (b) Schematic graph of experiment setup. (c) Schematic of flow chamber. (d) Definition of coordination systems for image processing, including local coordinate system xmO2ym along the migration direction and xfO2yf along the flow direction. The dotted and solid lines indicate the contours of a cell at its former and later location during migration. (e) Image processing for determining the parameters of cell migration and [Ca2+]i distribution under fluid flow. In the [Ca2+]i distribution graph, the red or green dots denote the points in the rear or front side along the migration direction and in the upstream or downstream side along the flow direction. |
2.3. Flow stimulation and [Ca2+]i measurements
2.4. Assessment of [Ca2+]i distribution during flow-induced cell migration
2.5. Statistical analysis
Table 1. The number of slides and cells in each group during 20 min. |
| Unidirectional flow | Slides | Cells | Oscillatory flow | Slides | Cells |
|---|---|---|---|---|---|
| No-flow | 3 | 12 | No-flow | 3 | 12 |
| Non-treated | 8 | 28 | Non-treated | 5 | 18 |
| TG | 5 | 16 | TG | 4 | 19 |
| U73122 | 4 | 18 | U73122 | 7 | 26 |
| Gd3+ | 3 | 16 | Gd3+ | 4 | 15 |
| Ca2+ free | 6 | 18 | Ca2+ free | 4 | 22 |
3. Results
3.1. Unidirectional flow increases the gradient [Ca2+]i distribution in the flow direction
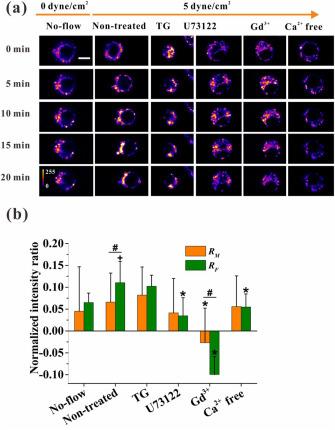
Fig. 2. Effect of unidirectional flow (5 dyne/cm2) on [Ca2+]i distribution and its regulatory signaling pathways. (a) Time-lapsed pseudo-color images of RAW264.7 cells under unidirectional flow with different treatments. Scale bar, 10 μm. (b) Normalized ratio of fluorescence intensity under no-flow or unidirectional flow with different treatments along the migration direction (RM) and flow direction (RF), respectively. Plus (+) represents significant difference between non-treated and corresponding no-flow group. Asterisk (∗) means significant difference with corresponding non-treated group, p < 0.05 (+, ∗, #). |
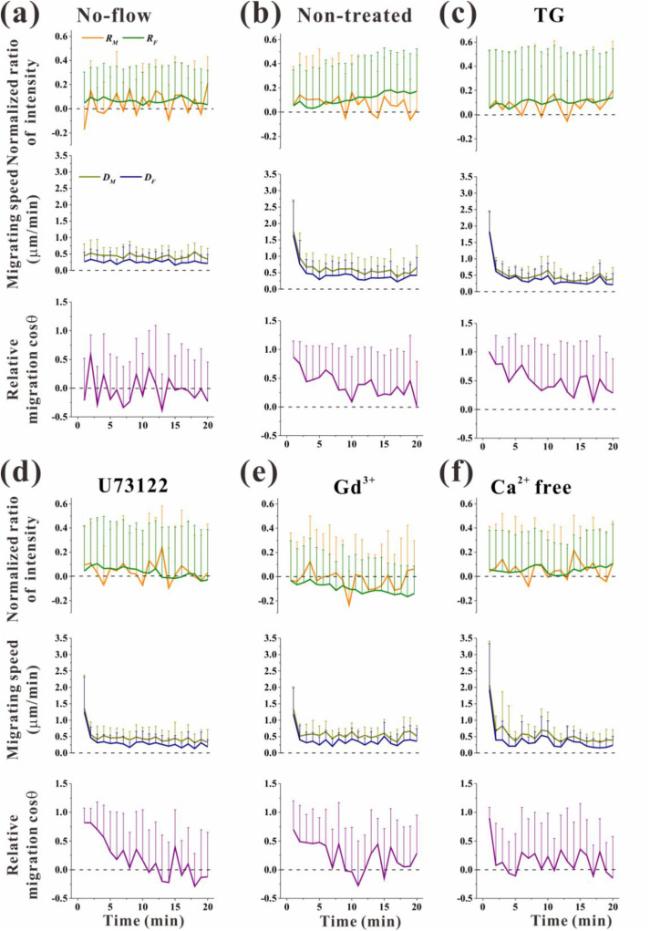
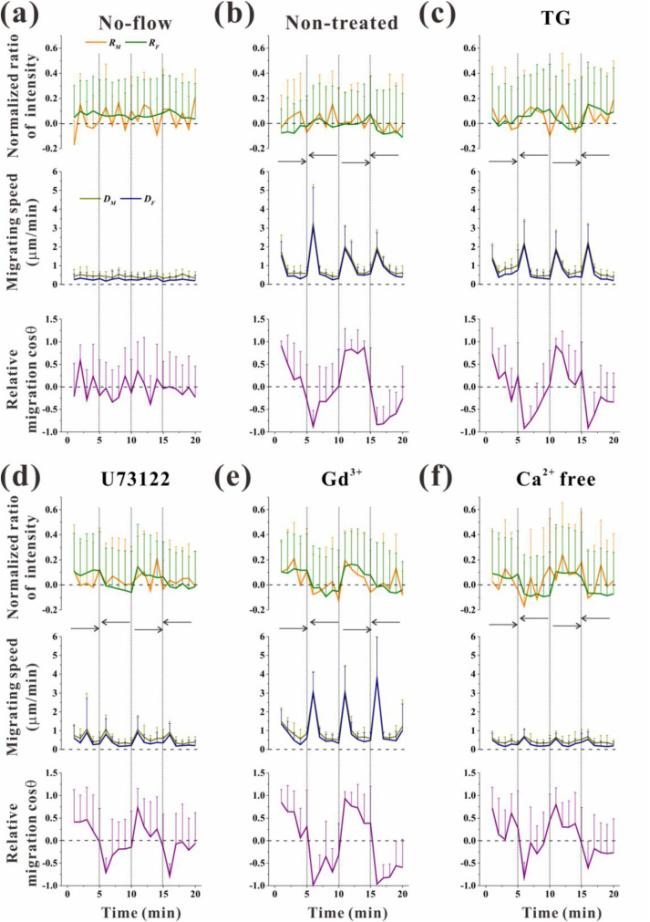
Fig. 3. Statistical analysis on the normalized ratio of fluorescence intensity, migration speed, and migration orientation of RAW264.7 cells under different treatments. (a) No-flow group. (b) Non-treated group, (c) TG group, (d) U73122 group, (e) Gd3+ group, and (f) Ca2+-free group under oscillatory flow (5 dyne/cm2). Each data point was obtained from at least 12 cells for each group every 1 min during 20 min unidirectional flow stimulation. |
3.2. MSCC/PLC regulates unidirectional flow-induced [Ca2+]i distribution
3.3. Oscillatory flow stimulates cell migration followed by adjustment of [Ca2+]i distribution
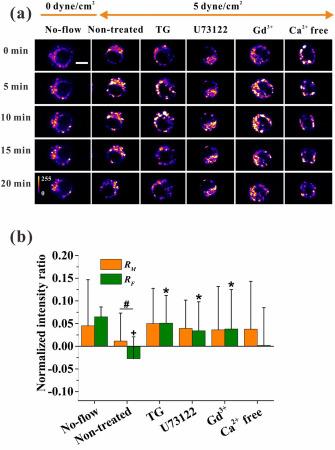
Fig. 4. Effect of oscillatory flow (5 dyne/cm2) on [Ca2+]i distribution and its regulatory signaling pathways. (a) Time-lapsed pseudo-color [Ca2+]i images of RAW264.7 cells under oscillatory flow with different treatments. Scale bar, 10 μm. (b) Normalized ratio of fluorescence intensity under no-flow or oscillatory flow with different treatments along the migration direction (RM) and flow direction (RF), respectively. Plus (+) represents significant difference between non-treated and corresponding no-flow group. Asterisk (∗) means significant difference with corresponding non-treated group, p < 0.05 (+, ∗, #). |
Fig. 5. Statistical analysis on the normalized ratio of fluorescence intensity, migration speed, and migration orientation of RAW264.7 cells under different treatments. (a) No-flow group, (b) Non-treated group, (c) TG group, (d) U73122 group, (e) Gd3+ group, and (f) Ca2+-free group under oscillatory flow (5 dyne/cm2). Each data point was obtained from at least 12 cells for each group in every 1 min during 20 min oscillatory flow stimulation. |
3.4. Extracellular calcium/MSCC/PLC/ER regulates oscillatory flow-induced [Ca2+]i distribution
4. Discussion
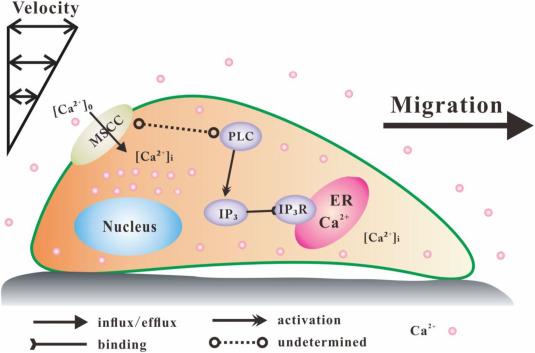
Fig. 6. Schematic of the FSS-induced migration of RAW264.7 cells and calcium signaling pathways. Osteoclast precursors move after being exposed to Unidirectional or oscillatory fluid flow and then the gradient distribution of [Ca2+]i can be found in the cells although long-lasting oscillatory flow may reduce the adjustment of [Ca2+]i distribution. This flow-induced [Ca2+]i distribution and cell migration is regulated by MSCC-PLC-ER pathway. |