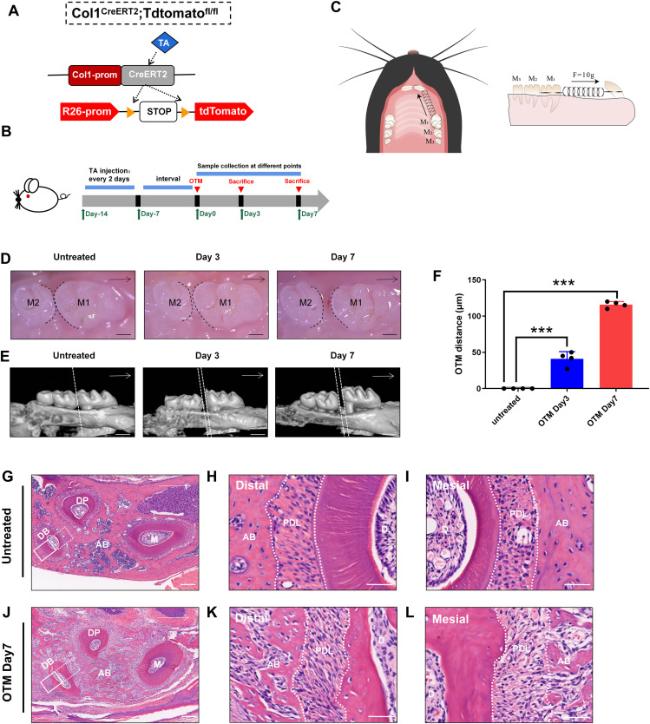
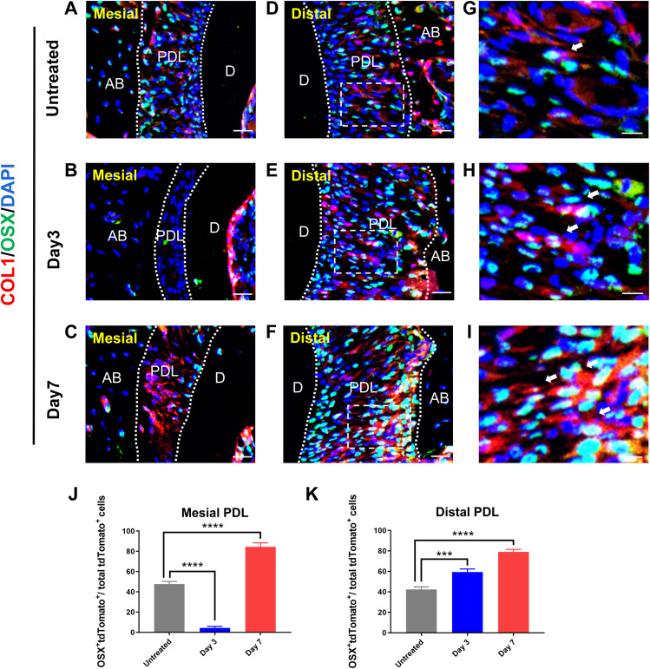
To investigate the dynamic distribution and differentiation potential of Col1-expressing cells during OTM, we created Col1-CreERT2/ROSA26-loxP-stop-loxP-tdTomato (
Col1-CreERT2; tdTomato) reporter mice. In these mice, Cre expression could be induced in Col1
+ cells at various developmental stages through the administration of tamoxifen (
Fig. 1A). To achieve the desired effects, we used a previously described induction strategy [
24]. Tamoxifen was intraperitoneally injected into 6-week-old control mice and Col1-CreERT2; tdTomato mice. The OTM model was initiated after a 7-day interval. The intact maxillary alveolar bone and tooth tissue were extracted for further analysis on days 0, 3, and 7 after euthanasia (
Fig. 1B and C). Both stereoscopic and micro computed tomography images revealed that the molars of mice that did not undergo orthodontic forces were precisely aligned and tightly interlocked. A small gap, measuring approximately 30-50 μm, between the maxillary first and second molars on day 3, was visible to the naked eye. On day 7, a gap covered by soft tissue was observed between the first and second molars of the mice. The distance between the molars was approximately 100-120 μm on day 7, thus indicating the successful construction of OTM model (
Fig. 1D-F). The mechanical force led to changes in both PDLs and alveolar bone tissue surrounding the maxillary first molars. We prepared paraffin sections from both OTM and untreated sides of the same mice on day 7 for HE staining, to obtain a comprehensive and accurate picture of the overall structure of periodontal tissues after mechanical force (
Fig. 1G-L). Examination of HE stained tissue sections indicated well-arranged alveolar bone tissue around the three roots of the maxillary first molar on the untreated side of the mice (
Fig. 1G). Furthermore, vertical sections of the roots revealed a dense and fibrous ring of PDL surrounding each root (
Fig. 1H and I). In contrast, increased formation of bone matrix between the three roots of the first molar was observed on day 7 of OTM. The newly formed bone tissues were disorganized, and the presence of many new blood vessels indicated a substantial change in the tissue structure (
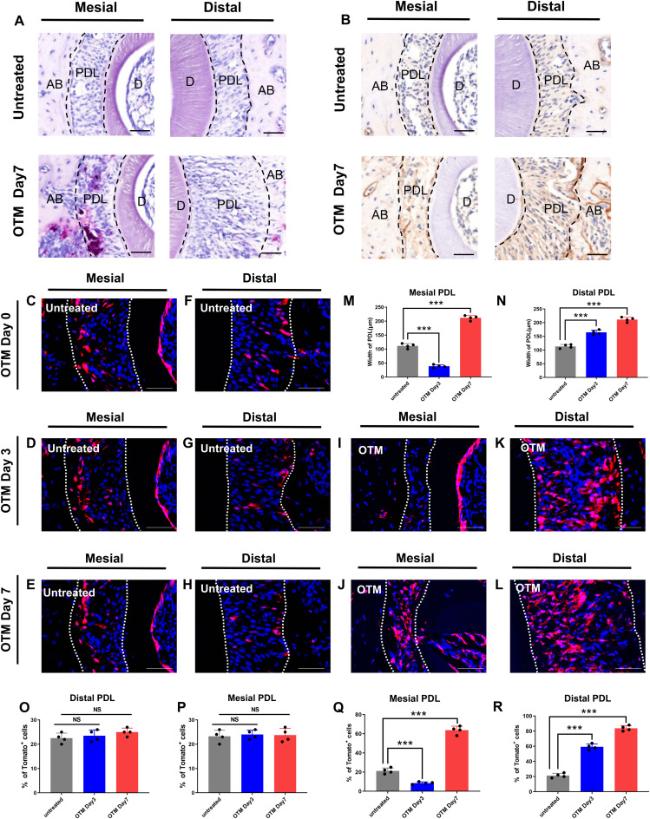
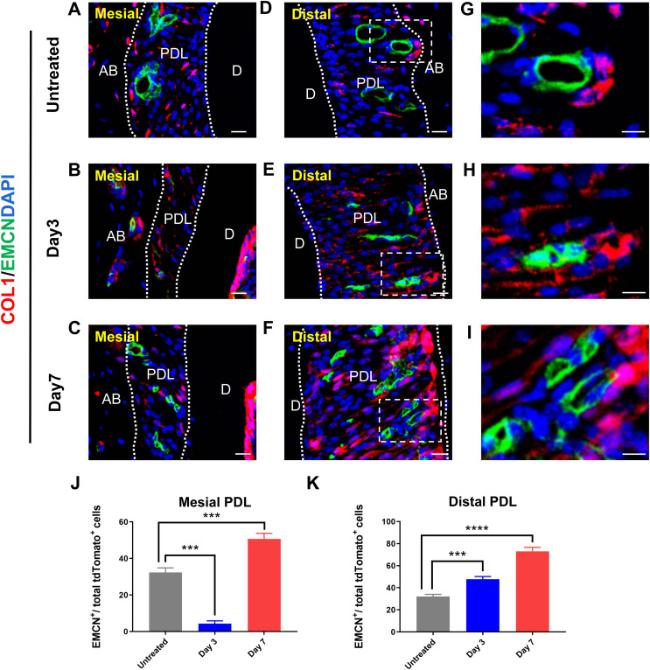
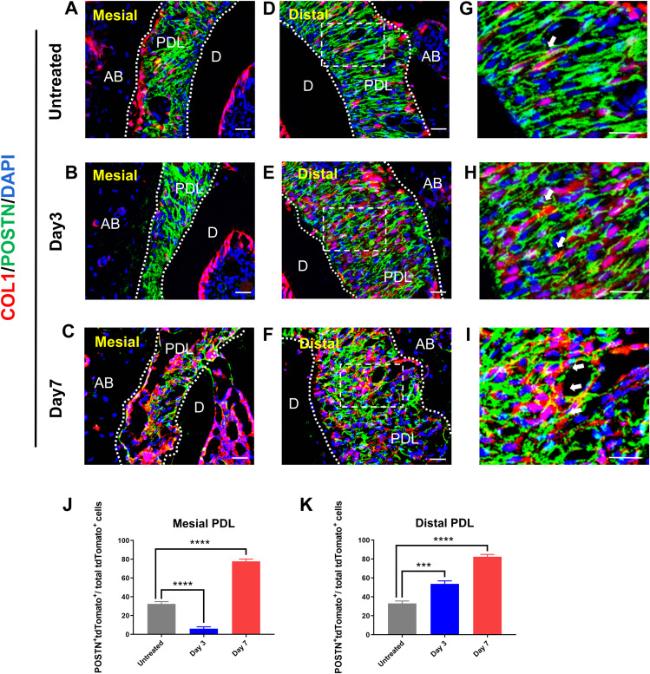
Fig. 1J-L). Meanwhile, width of the mesial PDL narrowed, and the fibers were compressed, whereas the distal PDL was stretched and widened (
Fig. 1K,
L). In brief, we successfully established an OTM model for controlling the interplay between osteogenesis and osteolysis by using Col1-CreERT2; loxp-tdTomato mice, which showed notable alterations in the periodontal ligament.