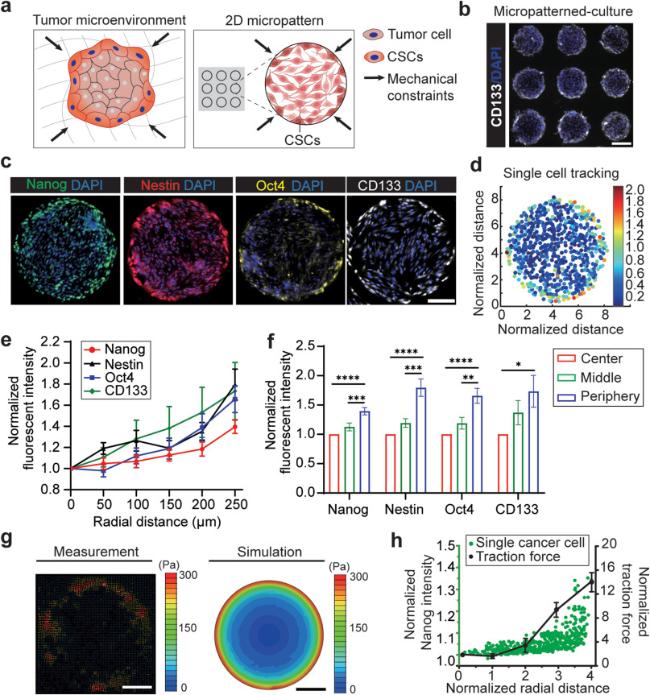
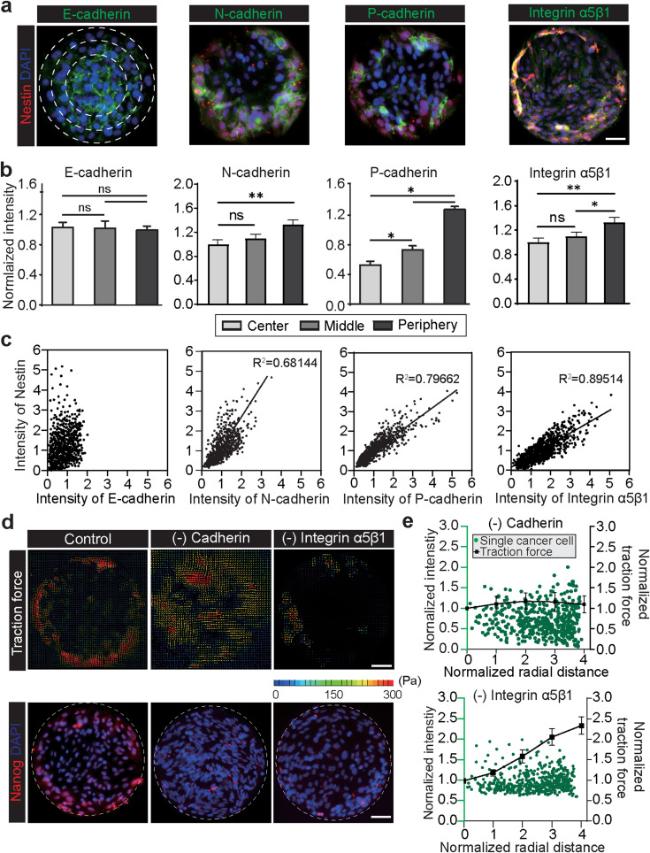
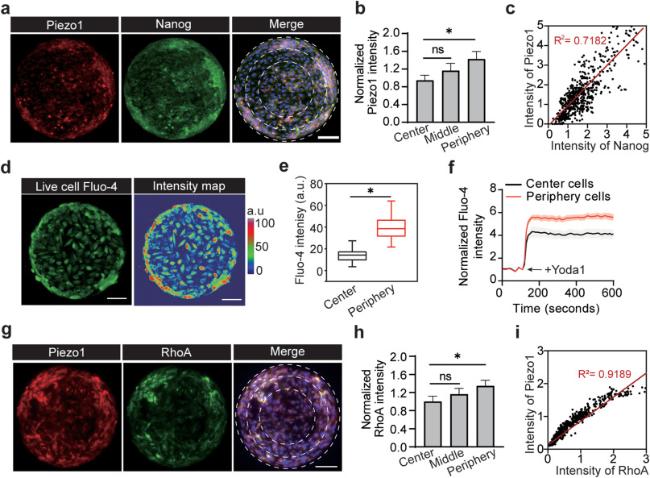
We explored how the interplay between the cell-cell interaction and cell-ECM interaction under mechanical constraints mediates the force-driven emergence and spatial patterning of CSCs in the geometric confined GBM cell colonies. As cells in the GBM colonies interact with surrounding cells and ECM, mechanical signals are transmitted by cell adhesive molecules including integrins and cadherins to actomyosin cytoskeleton, thus regulate cellular forces and downstream mechano-responsive signaling [
33] and mediate CSC phenotype in GBM [
37,
38]. We conducted immunostaining to assess the expression of cell adhesion molecules that are crucial for cell-cell adhesion (E-cadherin, N-cadherin, P-cadherin) and cell-ECM adhesion (integrin α
5β
1) (
Fig. 2a). The results showed that the expressions of N-cadherin and P-cadherin increased significantly from the center to the peripheral regions of the patterned cell colonies, while E-cadherin expressed uniformly in the colonies (
Fig. 2b). Such findings were expected, since E-cadherin expression has been found to be scarce in glioma cells, while N-cadherin upregulation has been widely exploited in glioma prognosis [
50]. In addition, integrin adhesome takes a critical role in transmitting mechanical signals from the ECM to the cytoskeleton and regulating actomyosin-mediated traction force exert by the cells on the ECM components [
51]. Previous studies have revealed that abnormal expression of integrin α
5β
1 in GBM cells results in a pathological alteration of cell-ECM interactions, and impact angiogenesis, migration, proliferation and therapy resistance of GBM [
52,
53,
54]. Our results proved that integrin α
5β
1 expression in the patterned cell colonies increased significantly from the center to the peripheral regions, which implicated the corresponding force gradients in the patterned GBM colonies (
Fig. 2a-b). The regression analysis between expression of adhesion molecules (E-cadherin, N-cadherin, P-cadherin, and integrin α
5β
1) and the CSC marker Nestin further showed that the expression level of Nestin in GBM cells in the colony is positively correlated with the expression levels of these adhesion molecules N-cadherin, P-cadherin, and integrin α
5β
1 but not E-cadherin (
Fig. 2c).