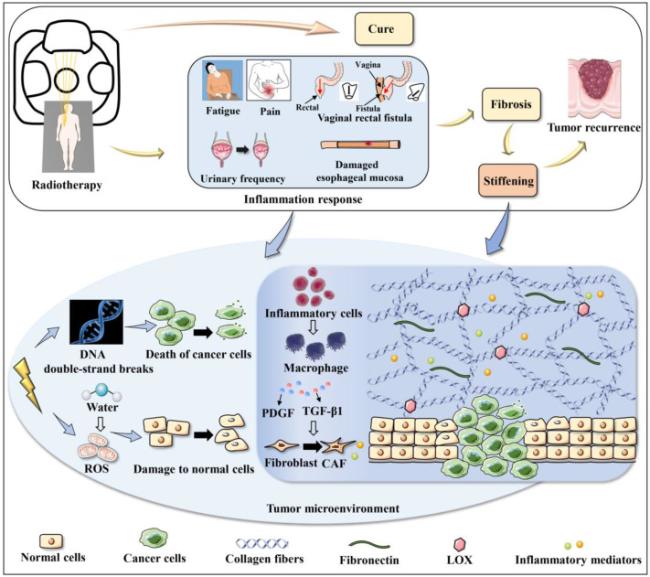
In addition to direct damage to the proteins and DNA of cancer cells, radiation also indirectly increases reactive oxygen species (ROS), which can lead to other cellular damage, as shown in
Fig. 1. These processes can lead to cell death or senescence after radiation [
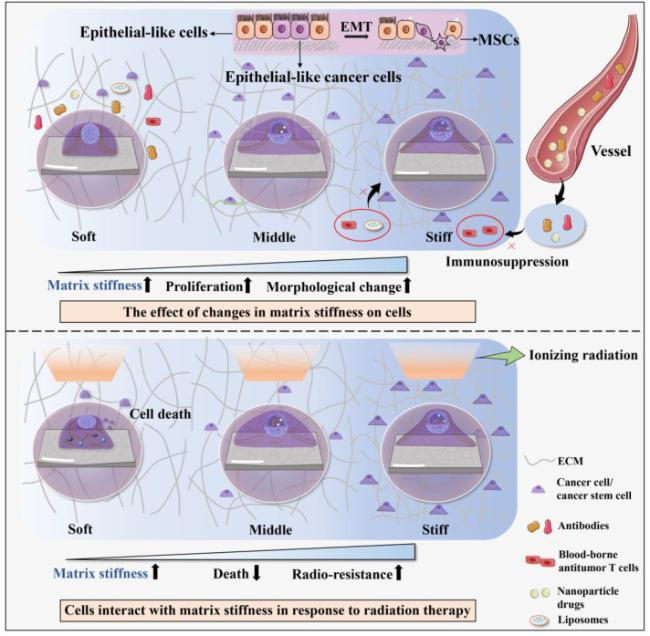
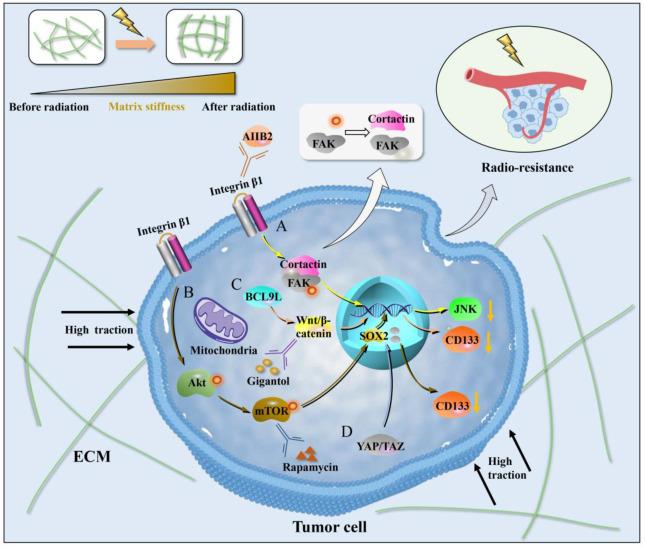
26], which can promote epithelial-mesenchymal transition (EMT), immunosuppression and tumorigenesis in neighboring cells, as well as re-entry into the cell cycle and activation of cancer stem cells, thereby promoting cancer cell survival [
27,
28]. Moreover, nitrogen species generated by IR can also lead to localized inflammation [
29]. Clinically, patients may experience various adverse reactions during or after treatment which related to inflammatory response, such as fatigue, pain in breast cancer radiation therapy [
30], vaginal rectal fistula in cervical cancer radiation therapy [
31], urinary frequency in pelvic radiation therapy [
22] and damaged esophageal mucosa caused by radiation esophagitis can also affect ingestion and acid reflux [
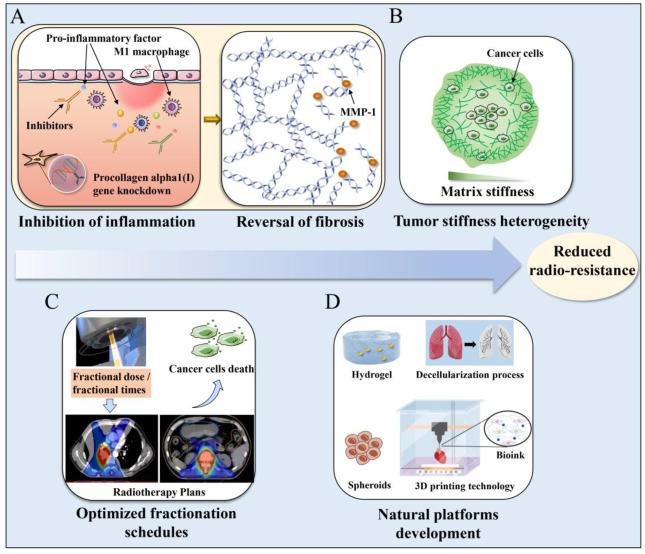
32]. If the inflammatory response is maintained, inflammatory cells such as neutrophils, lymphocytes, and monocytes arrive at the site of injury which resultants M2 macrophages produce platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1) and other pro-inflammatory factors, leading to recruit and stimulate the transdifferentiation of normal fibroblasts into cancer-associated fibroblasts (CAFs) [
33]. CAFs secrete inflammatory mediators, excess collagen and other ECM components to remodel ECM [
29]. In addition to change the amount of deposited collagen, the alignment of the collagen fibrils is also contributing significantly to the alteration of the matrix stiffness. The collagen matrix alignment
in vivo can significantly improve the strength and stiffness of entire matrix due to its better structural order relative a matrix that is a disordered matrix. For example, the study found significant differences in elastic modulus between the aligned and random collagen type I scaffolds, with the aligned groups (567 ± 134 kPa) having significantly greater elastic modulus than random groups (349 ± 85.5 kPa). Besides the variation of orientation of collagen fibrils during the pathological progression of tumors, the change of collagen organization was also found to affect matrix mechanics [
34]. Thickened and organized collagen fiber bundles are present in stiff tumor microenvironment. Besides, radiation promotes the secretion of LOX by cancer cells in a hypoxia-dependent manner which may lead to further cross-linking of collagen. These factors together result in the changes in ECM stiffness (
Fig. 1). Dense ECM and abnormal vessel formation which contribute to tumor hypoxia increase the number of hypoxic cells and the difficulty of treating surviving cells [
26,
35,
36,
37].