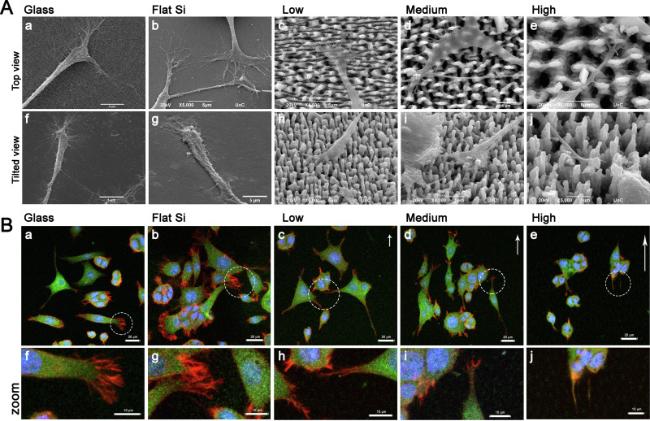
Initially, the morphology of PC12 cells, cultured on the different substrates and continuously treated with NGF for 24 h, was analyzed by SEM (
Fig. 2). Low magnification images (
Fig. 2A) on glass coverslips (control substrate) (
Fig. 2A a,f), flat (
Fig. 2A b,g) and low roughened Si (
Fig. 2A c,h) showed that PC12 cells are well spread on the substrate surface, while the cell somas show the normal polygonal shape. On medium roughened substrates (
Fig. 2A d,i), most of the cells appear to be bipolar, while on high roughened ones (
Fig. 2A e,j) less elongated and more bulged cells are observed. On glass, flat and low roughened Si, cell neurites appear to be long, with extensive branching toward multiple directions. However, as the roughness increases, smaller and fewer neurites are formed. Hence, on high roughness most of the cells show a few small or no neurites at all. Higher magnification images (
Fig. 2B) showed that on high roughness the cell somas are laying on top of the microcones but as the rest of the cytoplasm slightly sinks in the space between them, small bulges are formed wherever the microcones tips are located underneath (white arrows in
Fig. 2B e, f and
g). On low roughness, the microcones have lower aspect ratio and this effect is limited, therefore the cell surface morphology appears smoother (
Fig. 2B c) like the surface of the cells on glass and planar Si (
Fig. 2B a and
b). On medium roughness, the aspect ratio is similar to the high roughness but the interspike distance is half, therefore the surface distortion is also limited (
Fig. 2B d). From the above observations it is clear that the PC12 cell differentiation process is altered and the cells morphology is remodeled due to the underlying substrate morphology. Neuritogenesis also seems to be heavily affected by the high degree of roughness. Numerous studies have shown that substrate topography can alter cell attachment, cytoskeletal structure and cell shape and that these alterations can affect downstream signaling pathways by altering cell behavior [
30,
31,
32]. Hence, although initially it was believed that the morphology of the cell is determined by its function accumulating evidence demonstrates that the opposite is also true: physical properties of the cellular microenvironment determine cell shape and lineage fate decisions and thus its function [
33,
34,
35]. Neuritogenesis, the process of neurite sprouting from the cell soma, is the first step towards adopting a mature neuronal morphology and relies heavily on growth cone formation and stabilization. Growth cones are very motile expansions at the distal end of all neurites, capable of sensing the environment and guiding the neurite [
36,
37,
38]. The inability of cells cultured on high roughness Si substrate to adopt this characteristic neuronal behavior, motivated us to investigate further the development of the growth cone of NGF-treated PC12 cells. Representative SEM images of the growth cones in PC12 cells cultured for 24 h on the different substrates used are shown in
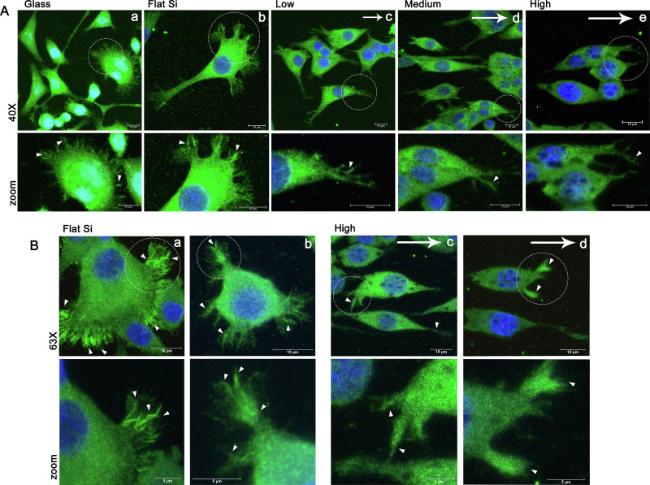
Fig. 3. Top (
Fig. 3A a-e) and tilted view (
Fig. 3A f-j) images were obtained for all samples tested. On glass and flat Si (
Fig. 3A a,b,f and
g) we observed the typical morphology of the growth cone with the palm-like structure of the lamellipodium and the finger-like protrusions of the pointed filopodia. The lamellipodium is firmly attached and spread on the substrate and ends up in a rich area of micro protrusions that freely extend and branch into multiple directions. On the low roughness (
Fig. 3A c and
h), the lamellipodium is wide and spread on top of the microcones with morphology similar to the ones observed on glass and flat Si. The many filopodia that are observed grow unhindered on the substrate and easily bridge the microcones' tips as they extend through the relatively small gaps throughout the discontinuous surface. On the medium roughness (
Fig. 3A d and
i), the lamellipodium is also spread on top of the microcones tips. The filopodia although are fewer either they are perched on the microcones' tips, or they are falling and wrapping around the microcones' bodies. On the high roughness (
Fig. 3A e and
j), the lamellipodium is narrow and cannot easily traverse from one microcone tip to the other, hence it grows only in the flat area between the base of the microcones. No filopodia are seen hanging between the microcones’ tips. The very few and short filopodia that are formed, are rather suspended instead of firmly attached, trying unsuccessfully to reach and adhere to the microcones. It is possible that the inability of filopodia to persist on top of the microcones tips long enough for the stabilization of the growth cone is responsible for the narrow shape of the lamellipodium and the decreased neurite sprouting and branching [
39]. Similar results were obtained with fibroblasts [
40] where the cell spreading, the lamellipodium area and the number of filopodia were reduced as the surface roughness increased. Moreover, it has been shown that the cell spreading of fibroblasts between deformable surfaces, such as flexible polymeric micropillars, is associated with increased cell contractility, actin cytoskeleton reorientation and cell and nucleus shape deformation [
41].