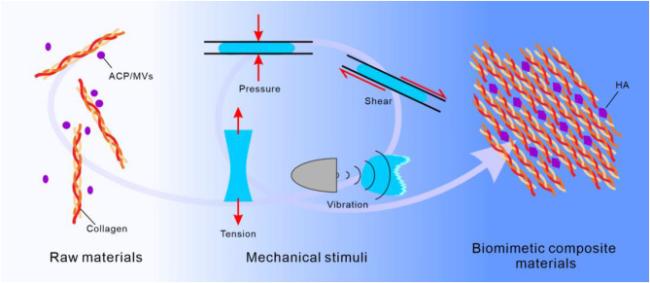
Biomimetic mineralization (BM) is a research approach that involves artificially mimicking the mineralization processes found in nature, with the aim of exploring ways to synthesize materials that closely resemble the structures and properties of natural biological materials [
1,
2]. The core of BM lies in understanding the mineralization mechanisms observed in the natural world, in order to create novel materials that may possess improved mechanical strength, biocompatibility, or other special characteristics. In BM, mechanical control plays a pivotal role, allowing for the adjustment of crystal orientation and morphology, regulation of crystal growth rates, and modification of the structure of mineralized composite materials. Several key mechanical stimuli, including pressure, shear force, tension, and vibration, have significant impact on the process (
Fig. 1) [
3,
4,
5]. Research in mechanically guided BM has found extensive applications in various fields such as biomedical engineering, regenerative medicine, and smart materials [
6,
7,
8]. Nevertheless, the development of more precise mechanical control methods to achieve desired mineralization processes remains a challenging task.
The history of mechanically guided BM research traces its origins to the observation and imitation of mineralization processes in the natural world. This field of study began with the understanding of how organisms utilize minerals in their structures, such as the calcification processes found in shells and bones. As scientific technology advanced, researchers embarked on endeavors to replicate these natural processes within laboratory settings, employing physical and chemical methods to control and direct material mineralization. This approach progressively expanded into various domains, including the development of novel materials [
9], biomedical applications [
10], and nanotechnology [
11]. These research efforts have not only deepened understanding of natural mineralization processes but also paved the way for the production of more efficient and sustainable materials.
Recently, Ayaza and coauthors introduced an innovative approach employing a mechanically-mediated reaction to enhance the mechanical properties of synthetic polymer composites [
12]. Addressing the challenges in achieving toughening comparable to biological mineralization in such materials, the researchers utilized the piezoelectrochemical effect of inorganic ZnO nanoparticles. Diverging from traditional mineralization processes, their technique involved the self-reactivity of spherical ZnO nanoparticles, resulting in the formation of microrods within an organogel and leading to a Zn/S mineral composition. This unique mechanistic reaction facilitated the selective deposition of mineral content within the composite, significantly augmenting its overall strength.
The article mentions that in future research, in addition to the current methods of stress application, the mechanisms by which other types of stress loading affect BM are also worth exploring, such as fluid shear stress (FSS). In our previous studies, we have delved into how FSS regulates the process of collagen mineralization [
13,
14]. As one of the key mechanical factors affecting collagen mineralization, the intensity and mode of FSS application have a significant impact. Research has shown that under FSS conditions of less than 1.5 Pa, the impact on collagen mineralization is positive. This is primarily reflected in the enhanced degree of collagen self-assembly, the accelerated formation and transformation of amorphous calcium phosphate (ACP), and the more orderly structure and arrangement of hydroxyapatite (HA). Additionally, under the influence of FSS, the size of ACP is effectively controlled, allowing mineral substances to be evenly distributed within collagen fibers, leading to intramolecular mineralization. These research findings not only contribute to a better understanding of the mechanism of natural bone tissue mineralization but also enrich the overall knowledge in the field of mechanobiology related to this process.
To more accurately simulate the FSS environment within the body, we developed a collagen mineralization system capable of providing periodic FSS, which we compared with the traditional method of mineralization induced by polyacrylic acid [
15]. Both periodic FSS and polyacrylic acid effectively control the size of ACP to prevent aggregation, thus facilitating intramolecular mineralization within collagen fibers. However, the distinction lies in the fact that, within appropriate cycles and intensities, periodic FSS not only accelerates the transformation of ACP into HA crystals but also mitigates the reduced transformation efficiency caused by polyacrylic acid. Under the influence of template analogs, periodic FSS further promotes the formation of highly oriented, hierarchically structured intramolecular mineralized collagen. These findings enhance the understanding of collagen mineralization mechanisms in natural bone matrix and offer invaluable guidance for the design of novel bone substitute materials with hierarchical structures.
Collagen mineralization plays a crucial role in various fields, especially in the realm of bone tissue engineering. Thanks to its exceptional biocompatibility, mechanical strength, and structural and compositional similarities to natural bone, mineralized collagen-based scaffolds are increasingly recognized as promising candidates for guided bone regeneration. We have developed an innovative method that involves electrospinning collagen solution combined with essential mineral ions, producing in situ mineralized uniform collagen-based scaffolds with superior osteogenic capabilities [
16]. These scaffolds demonstrate a uniform distribution of HA crystals within the electrospun fibers, significantly enhancing their tensile strength compared to pure collagen scaffolds and those with directly added nano-HA particles. Moreover, the in situ mineralized uniform collagen-based scaffolds exhibit improved cell compatibility, cell migration rates, and osteogenic differentiation properties. Therefore, these scaffolds not only retain the traditional function of inhibiting fibroblast invasion but also possess excellent osteogenic differentiation characteristics, providing an effective alternative for guided bone regeneration applications. In addition to our primary investigational endeavors, we have undertaken an in-depth synthesis and critical analysis of several pivotal topics, including “Biomechanics and mechanobiology of the bone matrix”, “Bioinspired mineralized collagen scaffolds for bone tissue engineering”, and “MV-mediated biomineralization mechanisms and treatments of biomineralized diseases” [
17,
18,
19]. These scholarly inquiries, meticulously collated into comprehensive review articles, have been disseminated through publication in renowned academic journals like “Bioactive Materials” and “Bone Research”. These contributions serve not only as a consolidation of current knowledge in these specialized domains but also as a catalyst for future scholarly discourse and research in the intricate fields of bone biology and regenerative medicine.
In summary, research into BM composite materials is of paramount importance due to their demonstrated potential across multiple domains. These include the development of novel biomimetic materials, unraveling the mechanisms of physiological and pathological calcification, and applications in regenerative medicine. Mechanical stimulation plays a pivotal role in maintaining the homeostasis of hard tissues within biological systems and is also a significant influencing factor in the process of in vitro BM. However, the precise control of mineralization through mechanical stimulation remains a significant challenge in the current research landscape. Future studies should place a greater emphasis on exploring various methods of mechanical stimulation for the precise control of mineral growth.