1. Introduction
2. Materials and methods
2.1. Animals
2.2. The establishment and groupings of the LPS infected shock rat model
Fig. 1. The process of animal model establishment and grouping. |
2.3. Video acquisition of microcirculation in rat mesentery
2.4. Analysis of flow pattern characteristics
2.5. Measurement of inflammatory cytokines
2.6. Statistical analysis
3. Results
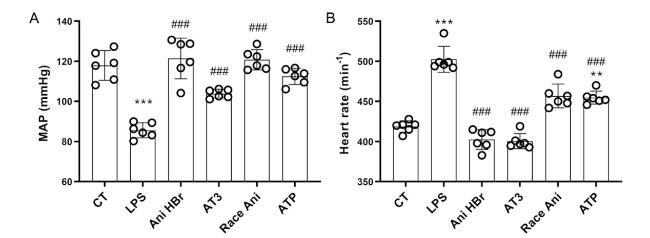
3.1. Improvement of MAP and HR in the systemic circulation of the LPS-induced septic shock rats
Fig. 2. Improvement of MAP and HR in the LPS-induced septic shock rats by Ani HBr and AT3. A. MAPs in the LPS-induced septic shock rats after Ani HBr, AT3, Race Ani and ATP treatments. B. HRs in the LPS-induced septic shock rats after Ani HBr, AT3, Race Ani and ATP treatments. ∗∗∗p < 0.001 vs. CT; |
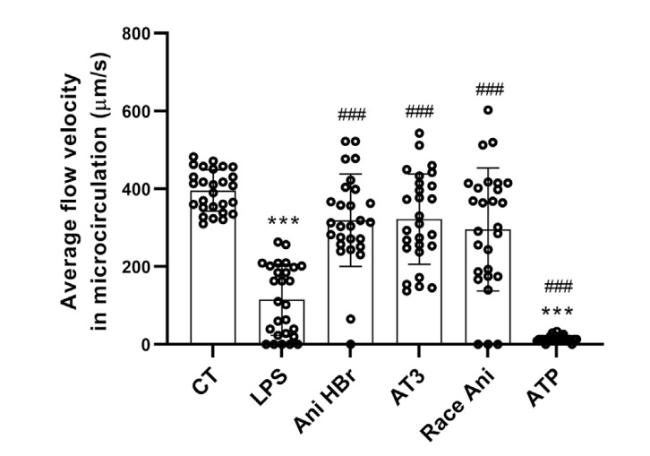
3.2. General characteristics of the microcirculation in sepsis and its treatments
Fig. 3. The velocity of mesenteric microcirculatory blood flow in the LPS-induced septic shock rats after Ani HBr, AT3, Race Ani and ATP treatments. ∗∗∗p < 0.001 vs. CT; |
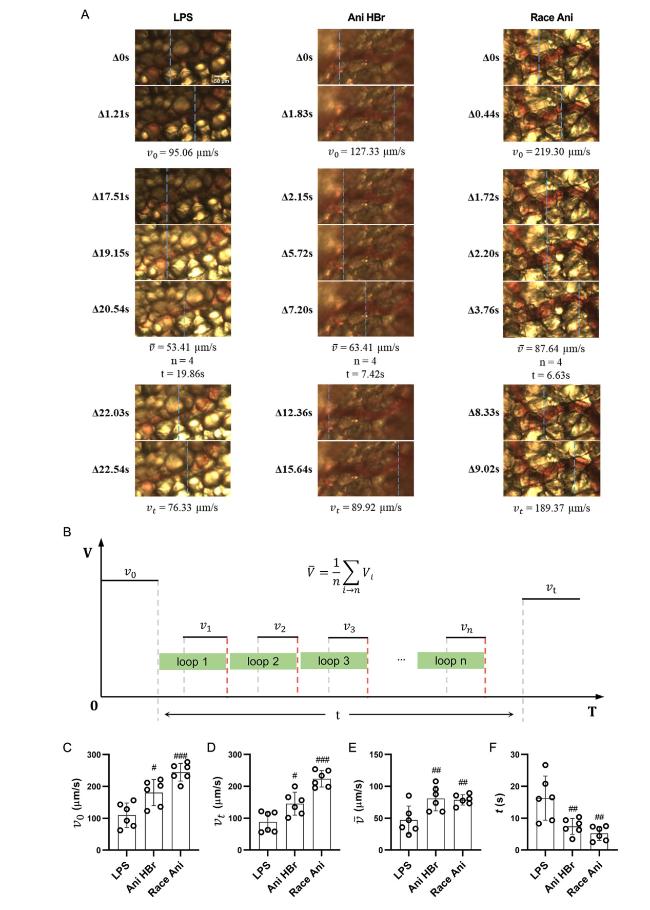
Fig. 4. The intermittent motion of blood flow in mesenteric microcirculation in the LPS-induced septic shock rats. A. the representative images of intermittent motion in LPS, Ani HBr and Race Ani groups. B. the parameters of intermittent motion. C–F. the average velocity of the blood flow at 1 s before stopping (v0), the average velocity of the blood flow at 1 s after the re-flowing (vt), the average velocity of the blood flow during intermittent motion (exclude the duration of cessation of blood flow) was ($\bar{v}$), and the total duration of intermittent motion (t) of intermittent motion respectively. #p < 0.05, ##p < 0.01, |
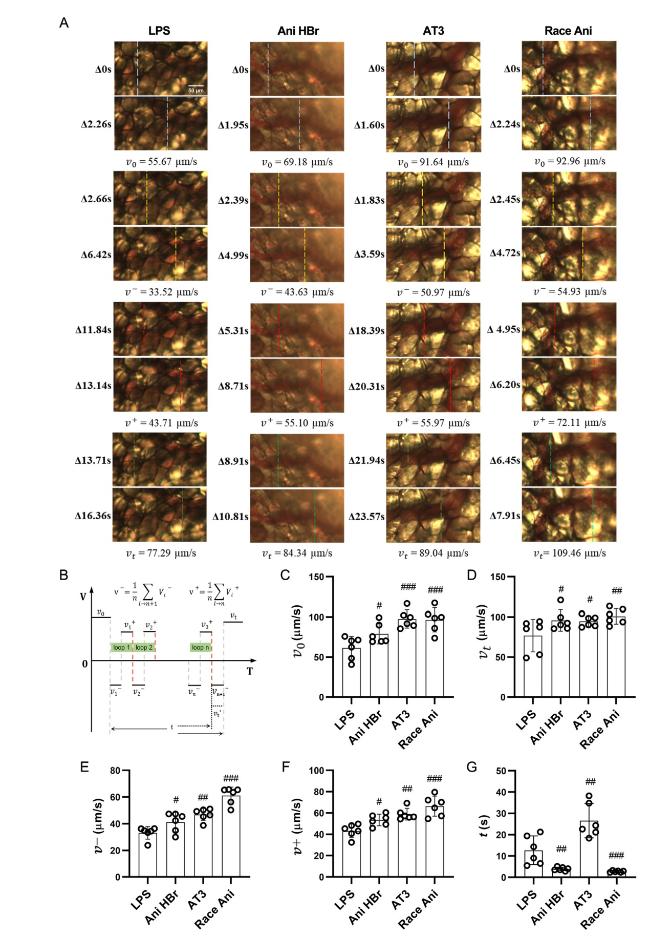
Fig. 5. The reciprocating motion of blood flow in mesenteric microcirculation in the LPS-induced septic shock rats. A. the representative images of reciprocating motion in LPS, Ani HBr and Race Ani groups. B. the parameters of reciprocating motion. C-F. the average velocity of the blood flow at 1 s before changing the direction (v0), the average velocity of the blood flow at 1 s after the re-flowing (vt), the average velocity in the back direction (v−) and forward direction (v⁺) and total reciprocal duration (t) of the reciprocating motion respectively #p < 0.05, ##p < 0.01, |
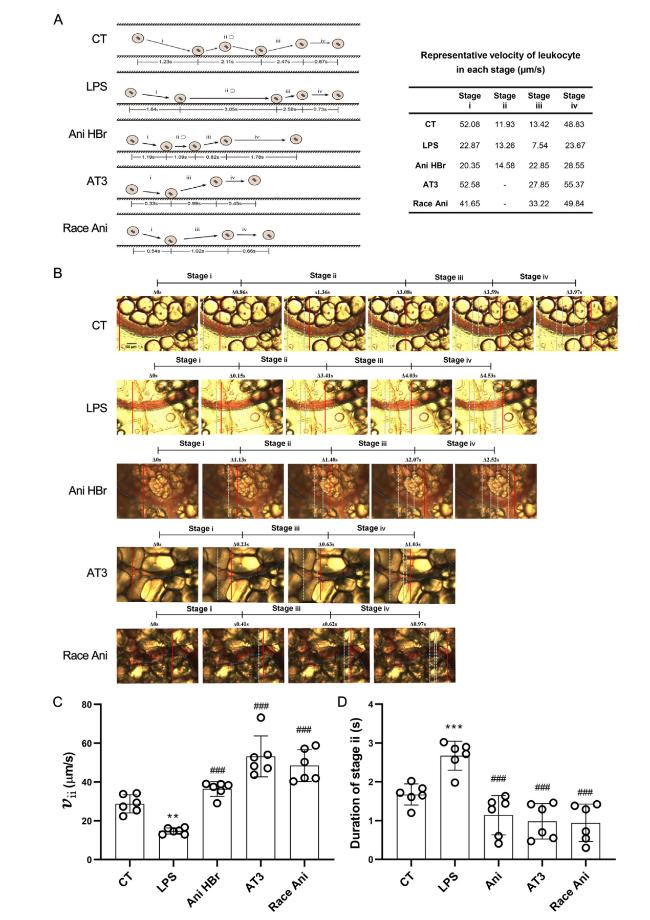
Fig. 6. The improvement of leukocyte dynamics in the mesenteric microcirculation in the LPS-induced septic shock rats. A. an illustration of leukocyte-endothelium interaction (left) with representative velocity (right) in each group. Stage i was leukocytes movement toward vascular wall. Stage ii was adhesion or rolling of leukocytes on vascular wall. Stage iii was detachment and ejection of leukocytes from vascular wall. Stage iv was movement of leukocytes along with the flow again. B. a representative image of leukocyte rolling on vascular wall. The solid lines indicate the immediate location of leukocytes (from Supplemental Video 2). C. the velocity of leukocyte rolling on vascular wall. D. the duration of leukocyte adhesion/rolling on vascular wall. ∗∗p < 0.01, ∗∗∗p < 0.001 vs. CT; ##p < 0.01, |
3.3. The intermittent motion of microcirculatory blood flow in sepsis and its improvement
3.4. Reciprocating motion of microcirculatory blood flow in sepsis
3.5. Leukocyte dynamics
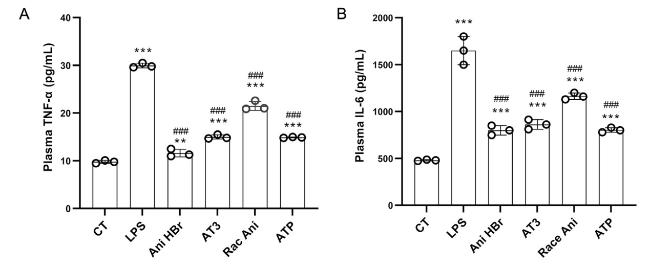
3.6. Inhibition of serum cytokines in the LPS-induced sepsis rat by Ani HBr and AT3
Fig. 7. The effect of Ani HBr, AT3, Race Ani and ATP on the plasma levels of TNF-α and IL-6 in the LPS-induced septic shock rats. A. TNF-α. B. IL-6. ∗∗p < 0.01, ∗∗∗p < 0.001 vs. CT; |