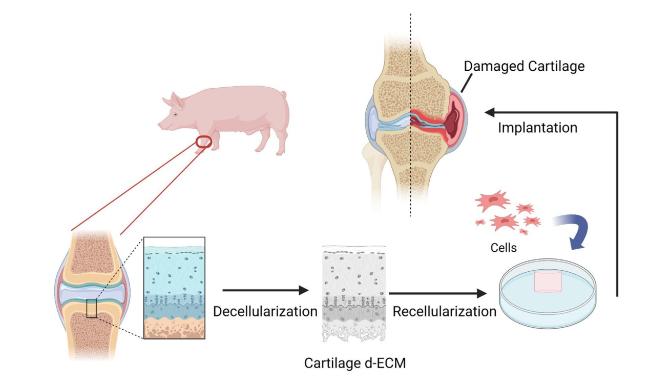
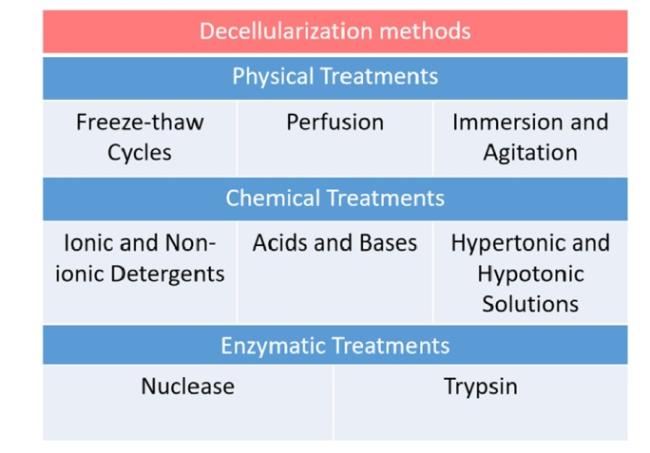
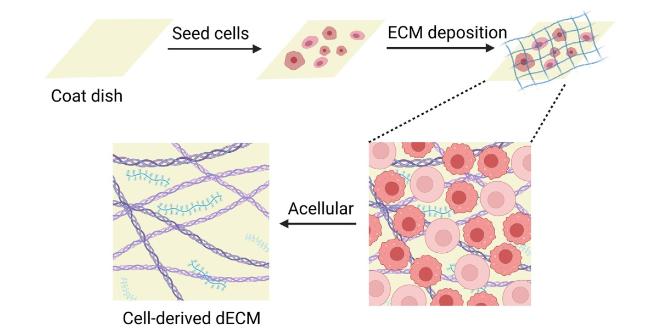
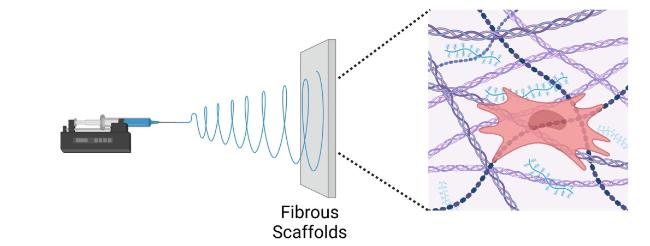
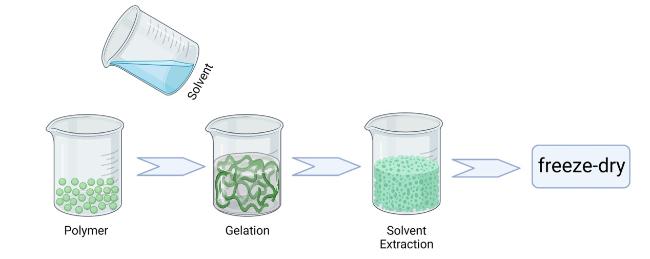
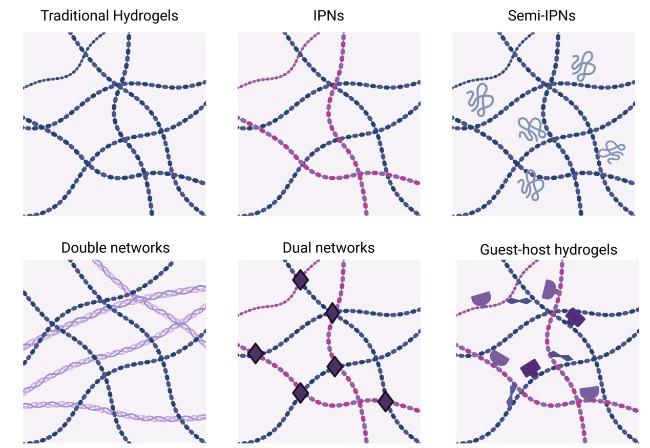
Hydrogels based on single-polymer networks usually exhibit very poor mechanical properties compared with native cartilage [
1]. With the aim of increasing the mechanical properties of hydrogels to approach those of hyaline cartilage, the focus is shifting from conventional hydrogels to multi-material hydrogel systems, often including two or more independent networks [
102]. Hydrogels based on interpenetrating networks (IPNs) are one of these multi-material hydrogels, comprising two or more separate crosslinked networks that partially intertwine [
103]. The schematic patterns of IPNs utilized in hydrogels are depicted in
Fig. 6 [
102]. Gupta et al. [
104] fabricated a 3D-printed scaffold with a self-healing IPN hydrogel-based construct with dual crosslinking capability using collagen, alginate and oxidized alginate, which caters to the load bearing requirements for total meniscus replacement as well as fulfils the micro environmental needs to provide a suitable niche for cellular growth and differentiation along with unique self-healing character that can heal after fractures. In addition to IPNs of two networks, tri-component IPNs were also investigated by Yu et al. [
105], who synthesized a novel biological degradable interpenetrating network hydrogel from gelatin, hyaluronic acid and chondroitin sulfate by Diels-Alder “click” chemistry, showing improved mechanical properties and great potential applications in CTE. Other types of multi-material hydrogels, including Semi-IPNs [
106], double networks [
107], dual networks [
108] and supramolecular hydrogels [
109], have also been applied in CTE, attaining stronger mechanical properties than networks of single polymers and exhibiting superior integration with surrounding tissue in vivo [
102]. Moreover, smart scaffolds that can respond to various stimuli have also been developed, enabling on-demand manipulation of cell microenvironments [
1]. Stimuli-responsive hydrogels, such as temperature-responsive hydrogels, pH-responsive hydrogels and light-sensitive hydrogels, have gained great attention in CTE due to their capability to undergo physical or chemical changes in response to external alterations [
110,
111]. Specifically, Moreira et al. [
112] synthesized a novel thermosensitive chitosan-based composite, chemically modified with collagen and reinforced by bioactive glass nanoparticles (BG), which resulted in a bioactive thermogelling composite with promising potential biomedical applications in tissue repair and regeneration.