The mechanical properties of biological tissues are critical for maintaining normal cellular behavior and tissue functionality. Previous studies showed that matrix stiffness significantly increases with age in different tissues, including the vasculature [
2,
3], brain [
4], and skin [
5]. Stiffened extracellular matrix (ECM) correlates with compromised stem cell niche, as well as dysfunction of tissue-specific stem cells. However, whether reducing the stiffness of the stem cell niche could reverse stem cell aging is still elusive.
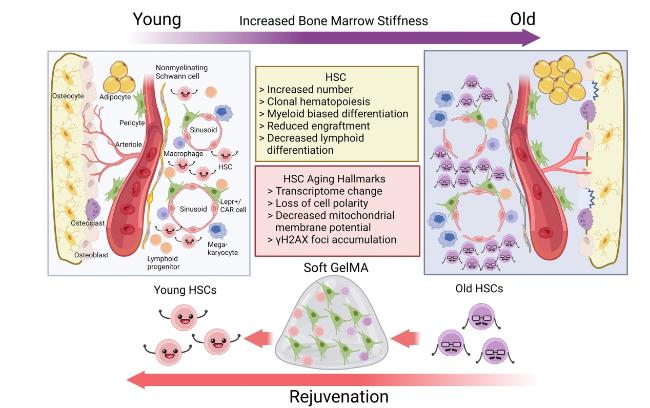
Hematopoietic stem cells (HSCs) site at the apex of the hematopoietic system, which can self-renew and differentiate into all types of downstream blood cells [
6]. During aging, HSCs are functionally impaired, which exhibit increased frequency (expand in number), myeloid-biased differentiation, clonal hematopoiesis, and reduced long-term reconstitution capacity. This could ultimately cause leukemia, anemia and dysfunction of the immune system (
Fig. 1). Whereas cell-intrinsic factors have been exploited to reverse HSC aging [
6], whether the HSC niche, namely, the bone marrow (BM) microenvironment, can be targeted for HSC rejuvenation remains largely unknown. The BM microenvironment is composed of different cell types, of which Leptin receptor (LepR)-expressing BM stromal cells (BMSCs) [
7,
8,
9] are the main source of niche factors indispensable for HSC maintenance [
10]. Notably, aging can also cause drastic change of the BM microenvironment, leading to compromised HSC function [
11] (
Fig. 1). Whether engineering a young BM organoid is a feasible way to rejuvenate HSCs has not been tested.
Recently, we have successfully reconstructed a soft BM organoid by 3D co-culture of HSCs and BMSCs in gelatin methacrylate (GelMA) hydrogel, and achieved robust HSC rejuvenation
ex vivo [
1] (
Fig. 1). To do this, we first detected the BM stiffness in young mice, and then titrated the concentration of GelMA hydrogel to reach similar stiffness. Remarkably, whereas 2D culture of BMSCs on plastic dish dramatically downregulated HSC niche factor (eg. Scf and Cxcl12), 3D culture in soft GelMA hydrogel significantly restored the morphology and niche factor expression in BMSCs. RNA-seq and ATAC-seq analyses showed that, as compared to 2D culture, 3D-cultured BMSCs were partially restored to their
in vivo state. Importantly, we found that Yap/Taz senses mechanical cues to promote BMSC proliferation and shift their cell fate during 2D culture, which was largely reverted upon 3D culture in soft GelMA hydrogel.
Next, we performed 3D co-culture of BMSCs and HSCs after medium optimization, and found that increased matrix stiffness (10% GelMA) significantly dampened the maintenance of HSCs ex vivo. Only in soft hydrogel (5% GelMA), HSCs co-cultured with BMSCs displayed enhanced multilineage long-term reconstitution capacity, with significantly higher level of lymphoid (T and B cell) engraftment. As myeloid-biased differentiation is an aging hallmark of HSCs, we went on to test whether aged HSCs could be rejuvenated using this novel co-culture system. By comprehensively analyzing the transcriptome, mitochondrial membrane potential, cell polarity, γH2AX foci and long-term reconstitution capacity, we demonstrated that aged HSCs (12- or 24-month-old) can be robustly rejuvenated to a level comparable with young HSCs (2-month-old) after 3D co-culture with BMSCs.
In summary, this study highlights the fundamental role of matrix stiffness in finetuning the BM hematopoietic microenvironment, and suggests remarkable plasticity of HSC aging. Intriguingly, bone marrow stiffening was identified as a novel aging hallmark of the hematopoietic system, which could be targeted to rejuvenate HSCs in vivo. Due to its simplicity and adaptability, the hydrogel-based BM organoid could be exploited to study cellular and molecular mechanisms by which hematopoietic cells and BM stroma interact, and to serve as an ex vivo disease model to dissect the pathogenesis of hematological disorders.