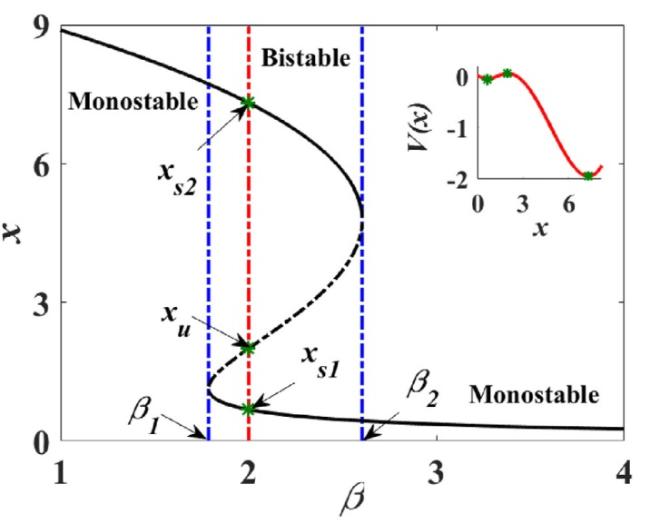
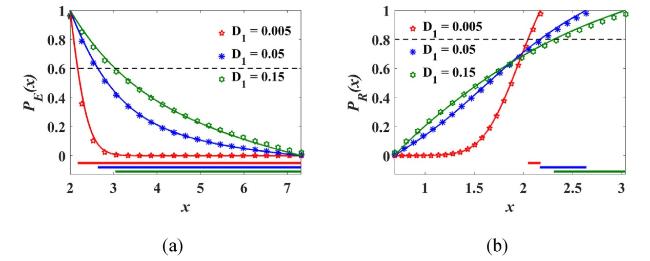
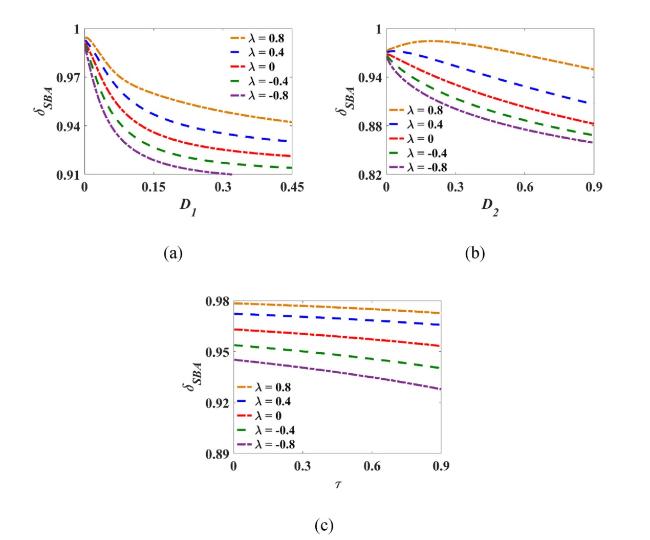
Noise-induced transition [
15,
16] usually refers to the qualitative changes in the state behaviors of the system induced by noise, which can be characterized by the change in the extrema of the SPDF (i.e., the emergence of new extremes or the disappearance of old extremes). Gaussian white noise has been widely applied for a long time for its advantage in facilitating mathematical processing and simplifying models. Gaussian white noise on its own fails to induce any effect on the extrema of SPDF when it is applied as an additive excitation. Moreover, the actual processes usually possess finite or even long correlation time, the
$\delta$-correlation of the Gaussian white noise as an idealization of the correlation of the actual processes fails to provide a good description of the real perturbations in nature. The colored noise with exponential correlation gives a more realistic description of the noise perturbation, and in the limit case where the correlation time tends to zero, the colored noise can degenerate into white noise [
17,
18,
19,
20,
21,
22]. With the deepening of research and experimental verification, it is found that the noise environment in practical applications is extremely complex and often presents non-Gaussian characteristics [
23,
24,
25,
26,
27,
28]. When the real perturbation deviates from the Gaussian distribution, the Gaussian-type assumptions appear to be out of practice. Numerous studies related to non-Gaussian noise have demonstrated that the presence of non-Gaussian noise can induce the system to behave in richer dynamics [
11,
29,
30,
31,
32]. Therefore, the study of tumor state transition under non-Gaussian noise has increasingly become a research hotspot. However, to my knowledge, few studies have focused on whether the presence of non-Gaussian noise will change the situation that independent additive Gaussian white noise can barely cause qualitative changes in the extrema. Hence, we carry out the analysis from this perspective and demonstrate that the non-Gaussian colored noise has indeed a substantial effect on the inability of Gaussian white noise on MPSS and thus contributes to the occurrence of transition induced by Gaussian white noise.