1. Introduction
2. PDX models from solid tumors for drug delivery and metabolism
Table 1. Establishment of humanized immune system mouse models. |
| Humanized models | Mouse strain | Establishment method | Ref |
|---|---|---|---|
| Hu-PBL-SCID mice model | SCID mice | Perihperal blood lymphocyte (PBL) cells were injected intraperitoneally or intravenously into non-irradiated or sublethal irradiated mice | [83,84] |
| Hu-HSC(Hu-SRC-SCID) mice model | NOD-SCID mice, NSG mice | 1. Human CD34+ hematopoietic stem cells (HSC) were injected into neonatal or adult immunodeficient recipient mice by intravenous injection or bone marrow cavity injection | [84,85,86] |
| 2. Human hematopoietic stem cells were intrahepatic injected into neonatal NSG or NOG-SCID mice irradiated with sublethal amounts to obtain good human cell transplantation and generate T cells, B cells, macrophages, NK cells and DC cells | |||
| Human BLT (bone marrow, liver, thymus) model | NOD-SCID mice, NSG mice | NOD/SCID mice irradiated with sublethal doses were transplanted with human embryonic thymus and embryonic liver tissue under the renal capsule, and CD34+HSCs were isolated from homologous embryonic liver by tail vein injection to construct a new humanized mouse model | [84,87,88] |
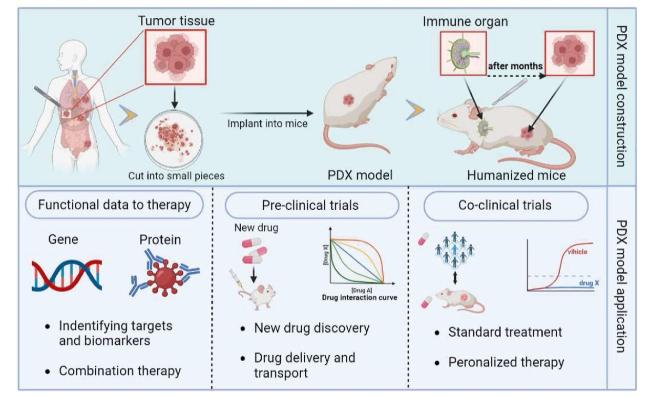
Fig. 1. Diagram showing the construction and application of the PDX models. |
2.1. Application of PDX model in drug delivery
2.2. Assessing drug metabolism by PDX models
3. CTC models derived from advanced-stage patients for personalized drug testing
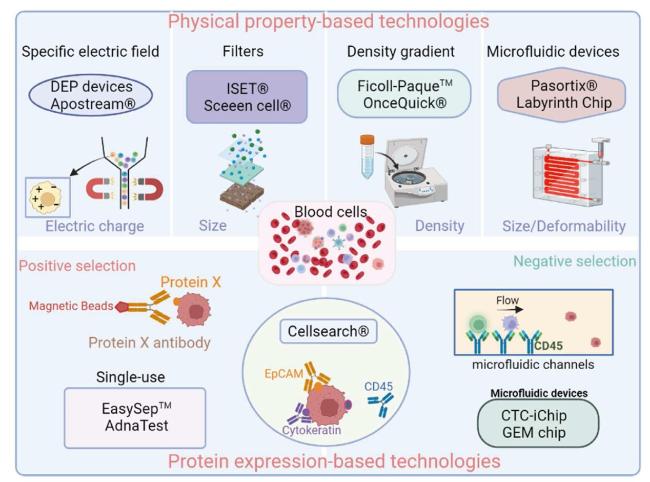
Fig. 2. Circulating tumor cell (CTC) enrichment techniques. |
Table 2. CTC cell lines in recent 5 years. |
| Cell Line | Type of Cancer | Culture Period or Passage | Specific Characteristics | Ref. |
|---|---|---|---|---|
| CTC-MCC-41 CTC- MCC-41.4 CTC-MCC-41.5 (A-G) | Colon cancer | Several cell lines from the same patient at different times (9 cell lines) | ALDH1 CD44 panCD66 EpCAM | [89] |
| CTC-TJH-01 | Non-small cell lung cancer | 24 months | CXCL5 CD44 ALDH1 | [90] |
| CTC-ITB-01 | Breast cancer | >4 years | E-Cadherin EpCAM K19 CD24 Twist 1 | [48] |
| UWG01CTC, UWG02CTC | Gastroesophageal cancer | >12 months | EpCAM(+) CK(+) CD44 E-Cadherin | [91] |
| EMC-Pca-41 | Prostate cancer | >1 year, 10 passages | TMPRSS2-ERG fusion loss of PTEN | [92] |
| Mel-167 PEM-22 Mel-182 PEM-78 | Melanoma | N/A | BRAF-mutant NG-2 MLANA | [93] |
Table 3. CTC-Derived Xenografts Models. |
| Type of Cancer | Model | Main findings | Ref. |
|---|---|---|---|
| Breast cancer | Dilution in Matrigel subcutaneous injection to node mice | Wnt pathway upregulation may be a potential therapeutic target in TNBC | [41] |
| Melanoma | Dilution in Matrigel subcutaneous injection into NOD.Scid IL2γ (NSG) mice | recapitulation of patient response to dabrafetinib in the CDX | [94] |
| SCLC | Dilution in matrigel/subcutaneous into NOD/SCID gamma (NSG) mice | ·1. Recapitulation of CTC genomic profile by CDX tumors | [79] |
| ·2. CDX mimicked donor's response to chemotherapy | |||
| Short-term non-adherent vivo cell cultures | Recapitulate genomic landscape and in vivo drug response | [95] | |
| NSCLC | Dilution in matrigel/subcutaneous into female CB-17/lcrHsd-PrkdcscidLystbg-J (SCID-bg) mice | Importance of mesenchymal CTCs with tumorigenic capacity | [96] |
3.1. CTC-derived Ex Vivo models for rapid drug testing of advanced-stage patients
3.2. CTC-derived xenografts for the evaluation of drug susceptibility
3.3. Maintain of the mutational traits of the primary tumor for personalized drug screening
4. Tumor organoids-on-chips for high-throughput drug screening
4.1. Construction of tumor organoids and organoids-on-chips
Table 4. Currently available tumor organoid models. |
| Cancer type | Source | Application | Ref. |
|---|---|---|---|
| Gastrointestinal cancers | Metastases | Simulate treatment response | [54] |
| Endometrial cancer | Endometriosis | Drug screening | [65] |
| Colorectal cancer | Primary tumor | Predict Chemoradiation Responses | [97] |
| Cervical cancer | Healthy cervical tissue | Model dynamics and viral oncogenesis | [98] |
| Lung cancer | Primary tumor | Therapeutic screening | [99] |
| Cholangiocarcinoma | Primary tumor | Evaluation of novel treatments | [100] |
| Prostate cancer | Metastases and CTC | Homeostasis, tumorigenesis and drug discovery | [101] |
| Liver cancer | Primary tumor | Disease modeling and drug screening | [102] |
| Breast cancer | Primary tumor | Captures disease heterogeneity | [66] |
| Pancreatic cancer | Primary tumor | Modeling and drug screening | [103] |
Table 5. Some reported organ-on-chip types. |
| Organ | Desired cell type | Ref. |
|---|---|---|
| Liver | hepatocytes, vascular endothelial cells, fibroblasts | [104,105] |
| Lung | alveolar epithelial cells, microvascular endothelial cells | [106] |
| Intestinal | Enterocytes, Adherent epithelium, Mononuclear Cells | [107,108] |
| Lymph node | Bulk B cells or naïve B cells, T cells and monocytes | [109] |
| Gut | Intestinal epithelial cells | [110] |
| Bone | Osteoblasts, Osteocytes | [111] |
| Breast | Mammary epithelial cells, Mammary fibroblasts, Vascular endothelial cells | [112] |
| Glomerulus | Kidney progenitor cells, human primary podocytes and glomerular endothelial cells | [113] |
| Eye | HUVECs, retinal pigment epithelium | [114,115] |
| Brain | dopaminergic neurons, astrocytes, microglia, pericytes | [116] |
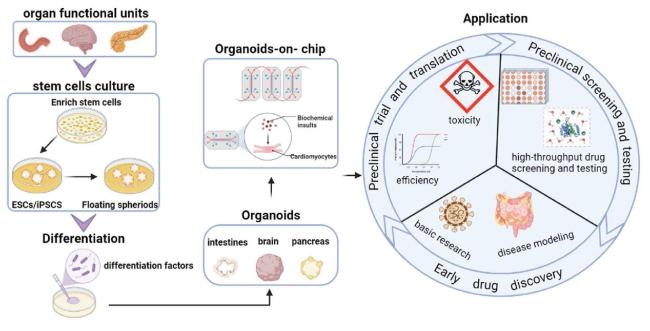
4.2. Application of the organoids and organoids-on-chips in drug therapy
Fig. 3. Construction and application of organoids and organoids-on-chips. |
4.3. Patient-derived cancer organoids high-throughput drug screening in different tumors
4.4. Organoids-on-chips for personalized drug testing
5. Discussion
Table 6. Advantages and disadvantages of three patient-derived tumor models. |
| Model | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| PDX models | • Recapitulate tumor heterogeneity | • Time-consuming and expensive | [9,82,117] |
| • Long-term stability | • Human-to-mouse xenograft differences | ||
| • Mimics the tumor microenvironment in vivo | • Ethical concerns | ||
| CTC-derived models | • Non-invasive sampling | • Low abundance | [118,119,120] |
| • Potential for liquid biopsy | • Heterogeneity | ||
| • Disease monitoring | • Lack of tumor microenvironment | ||
| Tumor organoids-on-chips | • Tumor microenvironment mimicry | • Simplified microenvironment | [121,122] |
| • High-throughput testing | • Technical challenges | ||
| • Personalized medicine potential | • Limited clinical validation |