Distraction osteogenesis (DO) has become a successful surgical method for endogenous tissue regeneration, particularly in the treatment of large bone defects [
1], non-union [
2], infection [
3], and limb deformities [
4]. Current DO techniques can be categorized into acute bone shortening and subsequent lengthening and bone transport, both of which rely on gradual distraction with use of external fixation to form new bone in the distraction gap [
5,
6]. These techniques of distraction osteogenesis have three distinct phases of treatment: latency, distraction and consolidation. Bone transport is more frequently used because of its advantages in maintaining limb length without causing soft tissue contraction [
7]. Despite many successful evidences, prolonged consolidation, docking site nonunion, and pin tract infection remain challenging complications in bone transport technique [
8].
As early as 1960s′, Dr. Wasserstein started to combine an intramedullary (IM) metallic nail, structural allograft and external frame to accelerate bone healing for patients of limb discrepancies [
9]. Later, the use of IM nails has been used in conjunction with an external fixator or inserted after lengthening has been achieved, to reduce fixator time and prevent regenerate deformity [
10]. Since 2000s’, implant innovation has led to the production of flexible intramedullary nails to avoid the use of external fixator [
11]. Our recent study published in
Biomaterials reported that a biodegradable magnesium IM nail significantly promoted bone consolidation in a rat femoral DO model [
12]. However, the functionality of a biodegradable IM nail with osteoinductive properties in bone transport remains a subject of inquiry.
Recently, Lin and coauthors innovatively developed an osteoinductive, biodegradable IM implant using a hybrid tissue engineering construct (HyTEC) technique as an adjunctive therapy for bone transport [
13] (
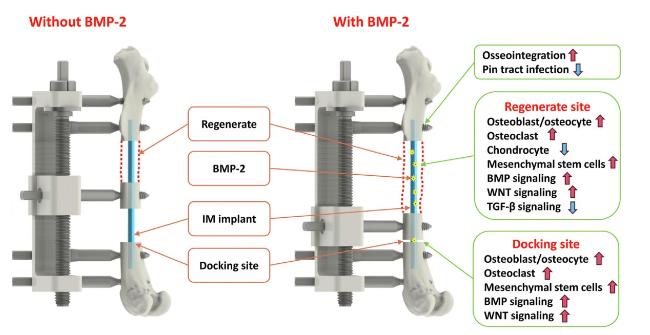
Fig. 1). The implant was coated with a controlled thickness of hydrogel containing bone morphogenetic protein-2 (BMP-2) for sustained release. The researchers investigated the efficacy of the implant in experimental models and compared it to standard treatments. There are four key findings as follows:
First, the osteoinductive and biodegradable IM implant was enabled by a newly developed hybrid tissue engineering construct (HyTEC) technique, which can coat the substrate with a controlled thickness of hydrogel containing biological substances such as cells or proteins while retaining porous structure. In this study, the authors fabricated a bioactive IM nail for sustained release of bone morphogenetic protein-2 (BMP-2) for this indication. To achieve a prolonged release of BMP-2, the authors applied additional PCL coatings onto the IM implant. Results showed that the amount of released BMP-2 after 28 days reduced from 84 % in the original IM implant to 62 % or 24 % when coated with one or three layers of PCL, respectively.
Second, the authors established a clinically relevant rat bone transport model which showed slow consolidation in the regenerate site and high non-union at the docking site. To demonstrate the translational potential of the HyTEC technique, the authors further established a preclinical sheep bone transport model using circumferential fixators as a proof-of-concept study. Distraction over the IM implant was successfully performed using the same protocol as that applied in the rat model, indicating the translational relevance of this HyTEC technique. In addition, the bioactive implant devices exhibited remarkable outcomes, with 100 % docking site union at as early as post-operation day 34 (POD 34), early weight-bearing recovery at POD 55, and no pin tract infections. In contrast, the surgical control and other treatment groups experienced high rates of docking site non-union, lack of weight-bearing capability, and pin tract infections.
Fourth, microarray data revealed remarkable elevations in the expression of osteoblast/osteocyte markers and chondrocyte markers within the regenerate on POD3, indicating that tensile strain actively promotes early-phase endochondral ossification. Conversely, the docking site exhibited significantly lower expression levels of these markers due to the absence of tensile strain and the presence of compression. This underscores the crucial role of tensile strain in stimulating bone regeneration, potentially involving mechanosensing and mechanotransduction signaling pathways within bone cells [
14,
15]. Additionally, a combination of tensile strain and eluting BMP-2 stimulated the expression of markers associated with bone formation, resulting in accelerated bone regeneration (
Fig. 1).
Overall, the study highlights the potential of the osteoinductive IM implant as an adjunctive treatment strategy to bone transport distraction osteogenesis for managing bone defects. By accelerating bone regeneration and minimizing complications, this HyTEC technique represents a promising solution for managing bone defects. Further research and clinical trials are warranted to fully explore the translational potential and broader applicability of this innovative approach.