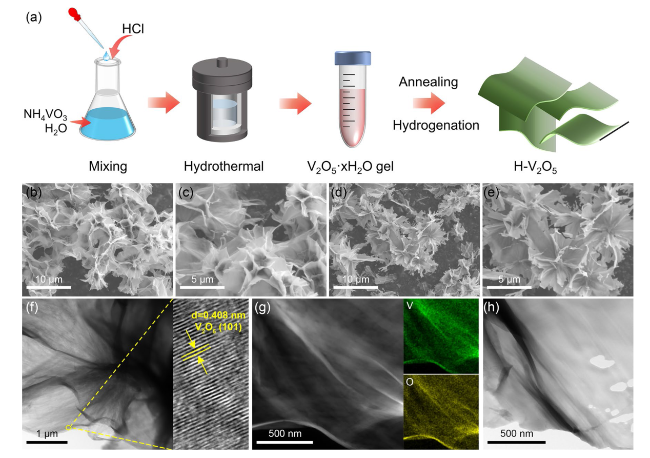
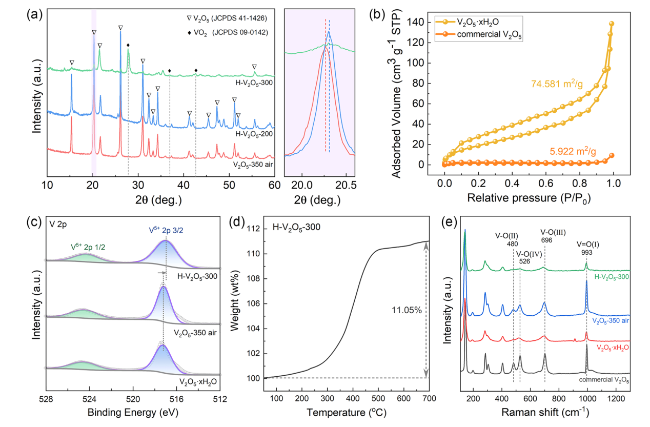
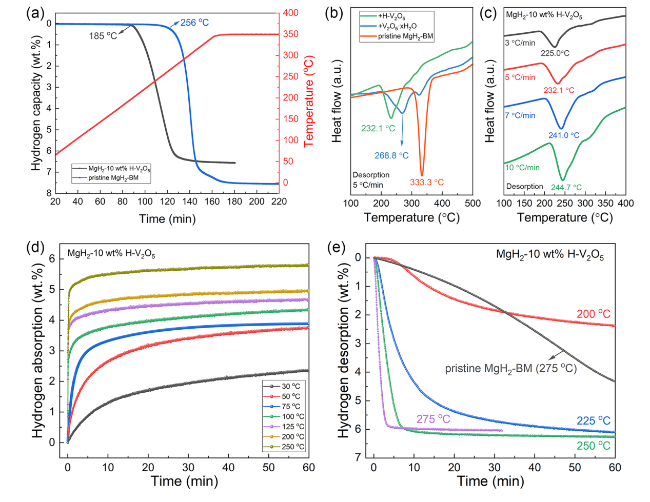
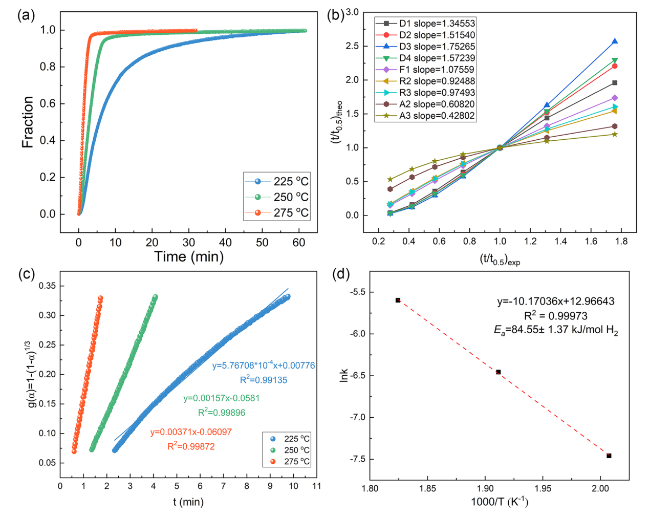
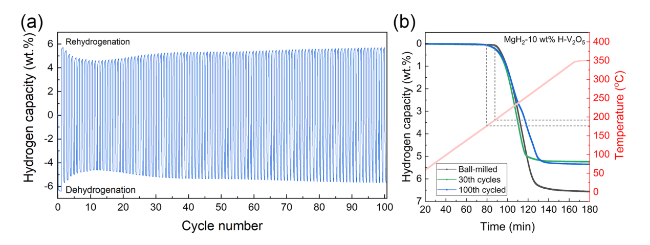
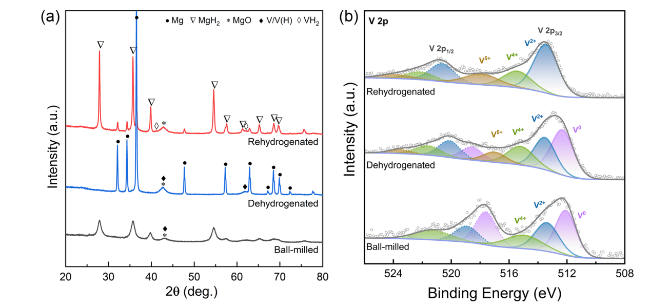
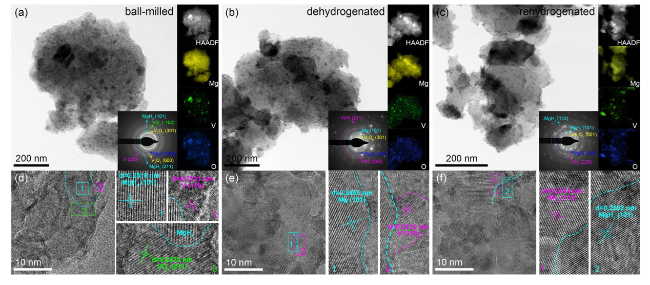
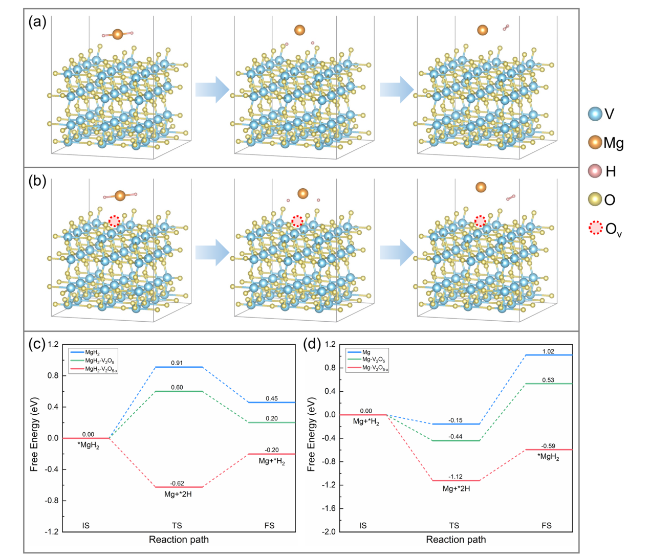
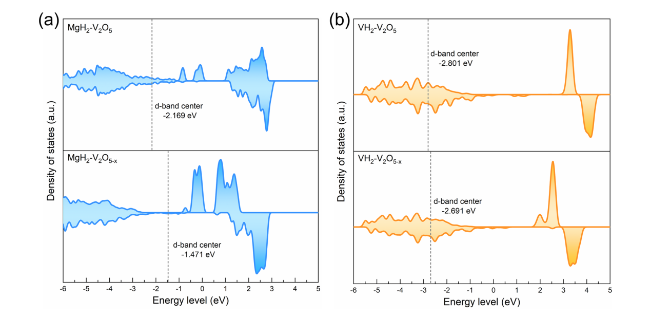
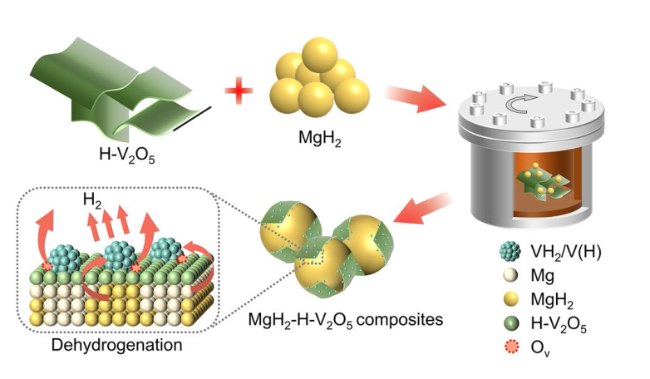
The catalytic effect of V
2O
5 nanosheets in improving hydrogen storage performances of MgH
2 is evaluated by mixing V
2O
5 nanosheets with MgH
2 through mechanical ball-milling. The temperature-programed desorption of H-V
2O
5-doped samples and pristine MgH
2-BM were first tested. As shown in
Fig. 3a, the pristine MgH
2-BM starts releasing H
2 at approximately 256 °C with a high terminal temperature at 350 °C, delivering a hydrogen desorption capacity of about 7.51 wt%, which agrees well with its theoretical hydrogen capacity. The results showed that the hydrogen release rate of the H-V
2O
5-doped samples was significantly faster than that of the pristine MgH
2-BM samples, especially at the beginning of the hydrogen desorption. The MgH
2-10 wt% H-V
2O
5 started to release H
2 at about 185 °C, with a hydrogen capacity of 6.54 wt%. Notably, the obvious decrease in the onset and terminal desorption temperatures of H-V
2O
5-doped MgH
2 compared to those of pristine MgH
2-BM indicates smaller particle size induced by the encapsulation effect from sheet-like structure of H-V
2O
5 and uniform distribution between H-V
2O
5 nanosheets and MgH
2 during ball milling process, which could be verified by the microtopography and elemental mapping results, hence promoting the catalytic effect of H-V
2O
5 nanosheets in improving hydrogen desorption performance of MgH
2. To further illustrate the superior catalytic activity of H-V
2O
5 nanosheets with oxygen vacancies towards MgH
2, the DSC curves of V
2O
5·xH
2O-doped MgH
2, H-V
2O
5-doped MgH
2, and pristine MgH
2 with different heating rates were compared (
Figs. 3b, c, S4 and S5). The peak desorption temperatures of the H-V
2O
5-doped MgH
2 decreased by about 36.7 and 101.2 °C compared to that of the V
2O
5·xH
2O-doped MgH
2 and pristine MgH
2, respectively, demonstrating the superior catalytic effect of H-V
2O
5 nanosheets composed of both V
5+, V
4+ and oxygen vacancies than V
2O
5·xH
2O nanosheets in improving the hydrogen desorption properties of MgH
2. According to the Kissinger's method [
53,
54], the apparent activation energies (
Ea) of MgH
2-10 wt% H-V
2O
5 (Fig. S4) and pristine MgH
2-BM (Fig. S5) were obtained. The results showed that the
Ea of MgH
2-10 wt% H-V
2O
5 and pristine MgH
2-BM were 113.8 and 137.4 kJ mol
−1, respectively. Thus, the addition of H-V
2O
5 nanosheets can significantly reduce the apparent activation energy of dehydrogenation for MgH
2 and improve the kinetics of the composites.