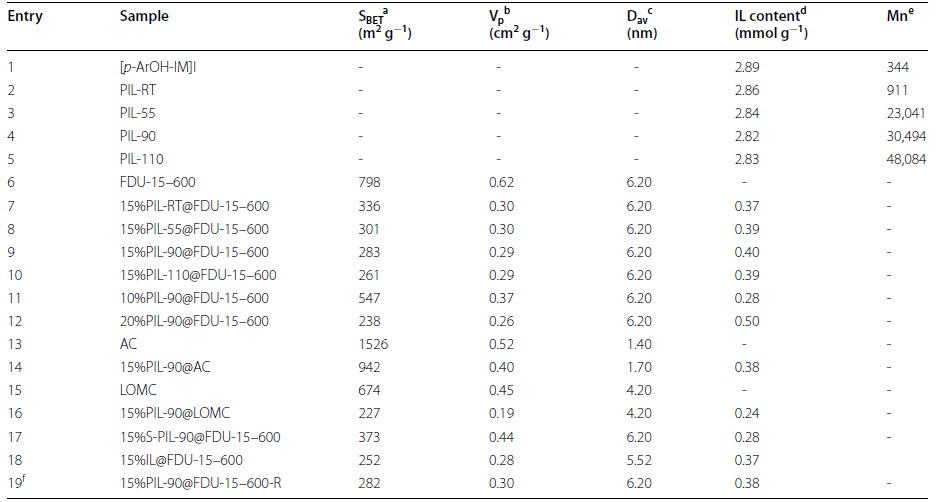
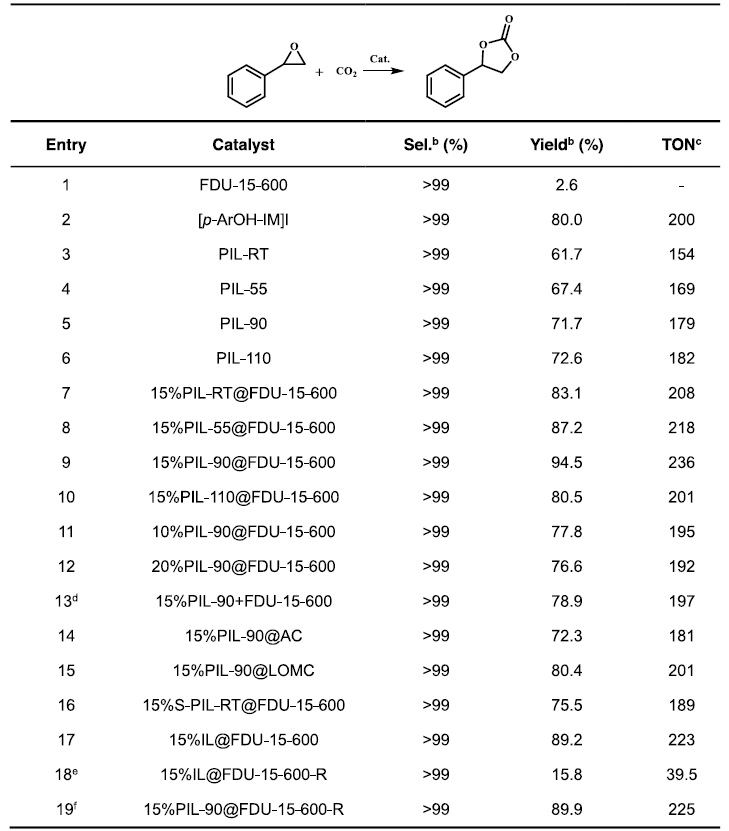
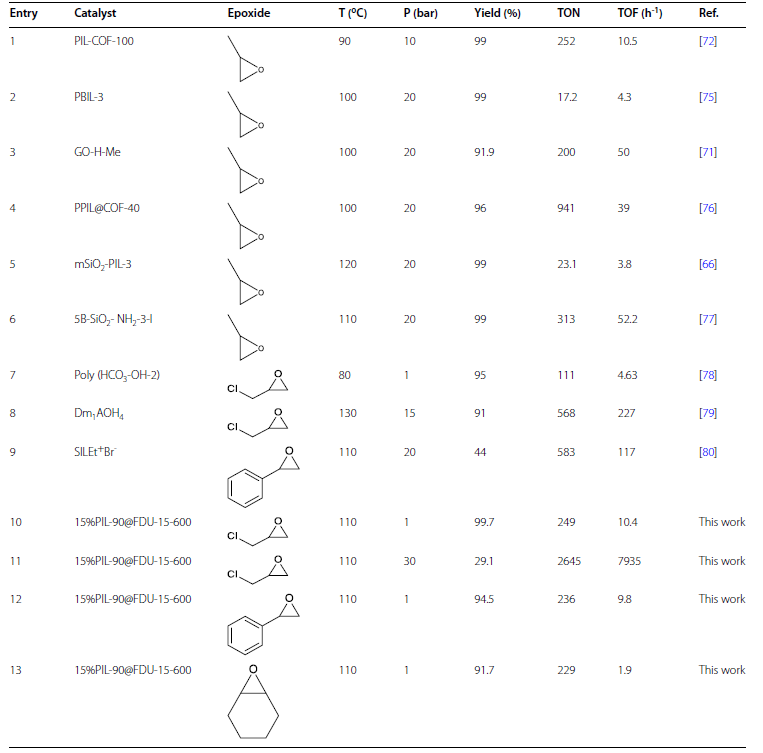
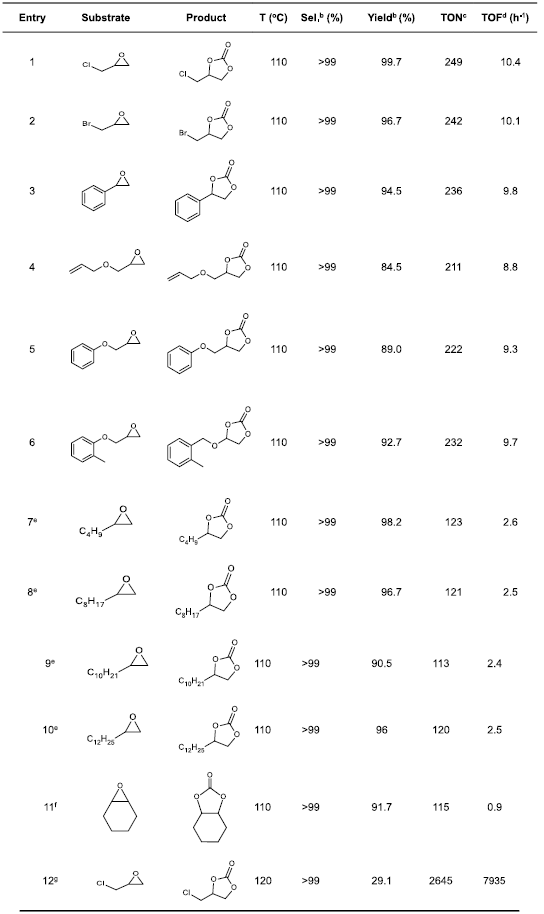
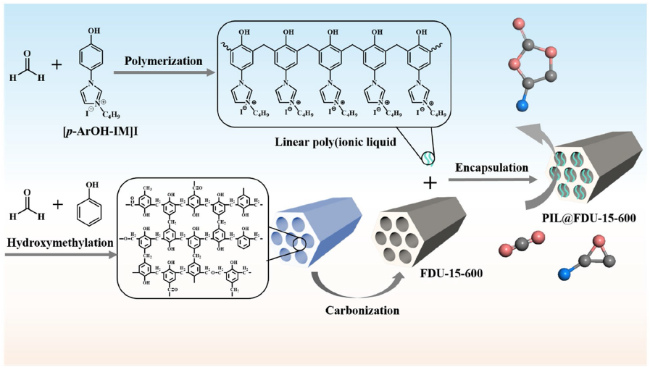
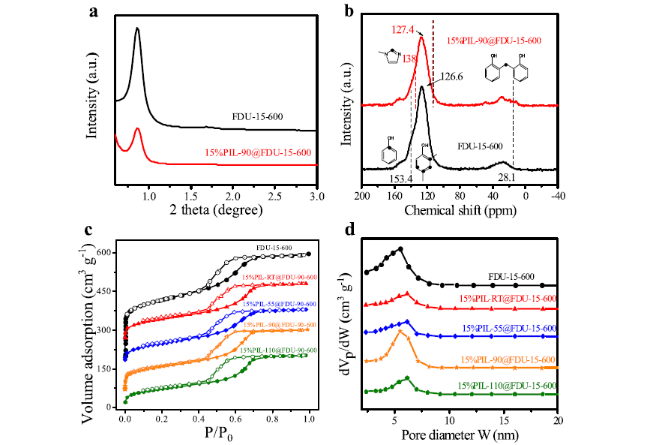
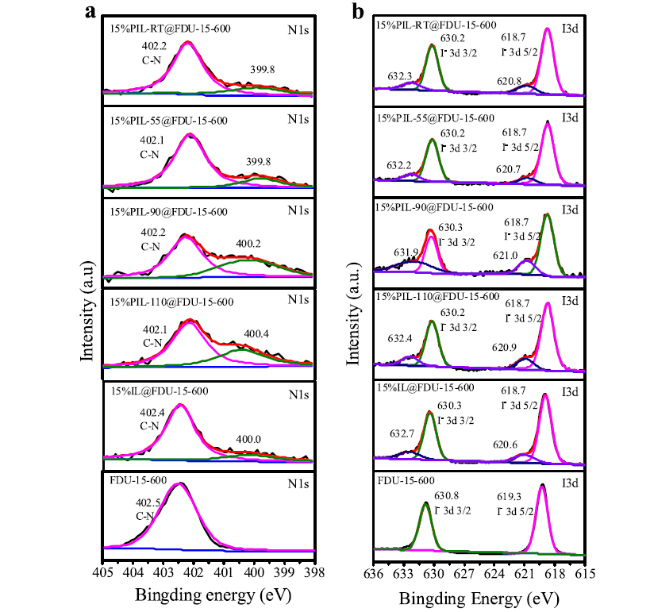
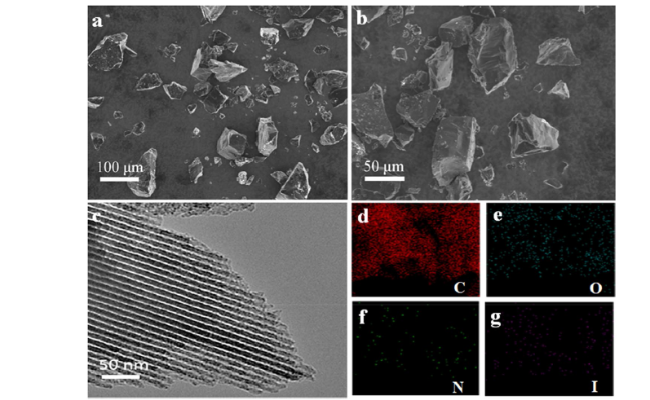
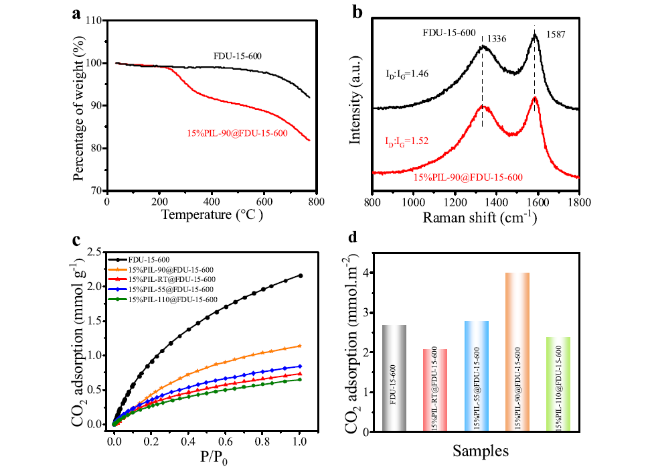
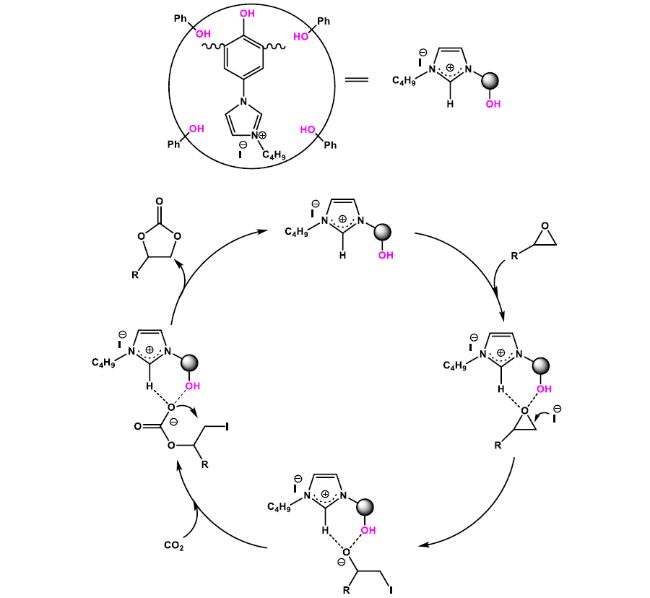
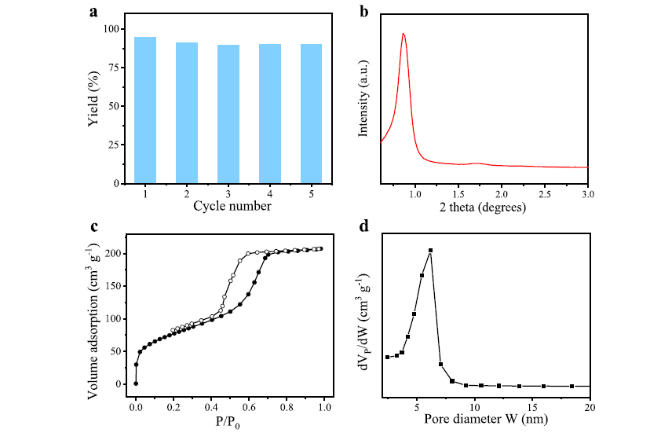
Cycloaddition of CO
2 with epoxides usually undergoes ring opening, CO
2 insertion, and ring closure [
37,
38,
39]. The first step is normally recognized as the rate-determining step and is triggered by an attack by a nucleophilic agent. The epoxide activation can be greatly promoted by Lewis acids (metal sites) [
24,
25,
26,
28,
29] or hydrogen bond donors (HBDs) such as the hydroxyl group [
5]. The anions of ILs were the efficient nucleophilic agents and the ILs’ structure associating with the corresponding catalytic function can be facilely modulated at a molecular level by integrating versatile functional groups to promote CO
2 affinity and epoxide activation. Therefore, ILs and their derivatives have been extensively investigated as a large family of environmental-friendly metal-free catalysts for CO
2 cycloaddition [
21,
40]. For example, phenolic hydroxyl group functional IL showed excellent activity in the CO
2 cycloaddition and achieved a high yield of 96% in the conversion of epichlorohydrin (ECH) at room temperature and atmospheric conditions [
33]. To facilitate catalyst recycling, great efforts have been made to develop IL-related heterogeneous catalysts mainly comprising two following strategies [
41,
42,
43]. One is the synthesis of ionic moieties containing polymers through the polymerization of IL monomer or with other linkers through various polymerization pathways such as free radical polymerization, hypercrosslinkage, and condensation [
44,
45,
46,
47]. For example, free radial self-polymerization of 2-(dimethylamino) ethyl methacrylate derived IL monomers afforded a series of PILs that can effectively catalyze the CO
2 cycloaddition with multiple epoxides with the yields of 70-98% at 110 °C and 20 bar [
46]. Bifunctional ionic polymers were directly synthesized through co-polymerization of tris (4-vinylphenyl) phosphine and functional dibromides and the resulting carboxyl-containing one exhibited the yield above 99% at 140 °C in the transformation of atmospheric CO
2 via cycloaddition with ECH [
47]. Alternatively, anchoring ILs and PILs on the porous materials provided a promising approach toward IL-derived solid materials that can inherit the abundant porosity of parent supports [
8,
45]. More importantly, the combination of surface groups in the supports and immobilized moieties favors reaching the highly effective synergistic conversion of CO
2 via cycloaddition with epoxide. Particularly, encapsulation of PILs on the ordered porous materials benefits to balance the stability and activity thanks to the confinement of the ordered porous channels and the spatial satisfaction of different reactive sites [
48,
49]. This strategy is feasible and versatile for IL solidification because it does not rely on the designation of polymerizable IL monomer for the pore formation during the polymerization process. Many ordered porous materials with the large surface area such as zeolites, MOFs, COFs, and mesoporous silica have been explored to immobilize ILs and PILs for the preparation of heterogeneous IL-derived catalysts [
16,
27,
28,
50]. Carbon materials with the features such as large surface area, tunable porosity, and abundant surface functional groups have been explored as the supports and catalysts in the CO
2 cycloadditions [
51,
52,
53,
54,
55,
56,
57]. For instance, as a metal-free heterogeneous catalyst, graphene oxide (GOs) achieved 90.3% conversion and 98.6% selectivity in converting styrene oxide to styrene cyclic carbonate at 140 °C and 1 bar by using N,N-Dimethylformamide (DMF) as the solvent [
55]. A PIL/graphene composite was prepared via an in-situ surface construction strategy and gave a yield of 99% in the coupling of 15 bar CO
2 with propylene oxide at 100 °C [
56]. Carbon supported single Zn atom catalyst was constructed from the straightforward carbonization of carbon supported phenanthroline-ligated Zn(OAc)
2 complex and exhibited the yields of 93-98% in the conversion of seven epoxides via coupling with 5 bar CO
2 at 100 °C in the presence of tetrabutyl ammonium bromide (TBAB) as an additive [
57]. The abundant surface oxygen groups such as hydrogen groups on the carbon materials can serve as effective HBD to promote the epoxide activation to accelerate the corresponding transformation into the target cyclic carbonates [
3,
41]. In addition to the disordered carbon materials mentioned above, ordered porous carbon materials such as CMK, FDU, and CGM-Cs (carbon-based Cornell Graded Materials) series [
58,
59,
60]. Nonetheless, the encapsulation of PILs into the ordered porous carbon materials is still to be explored for the preparation of metal-free heterogeneous catalysts for CO
2-epoxide coupling.