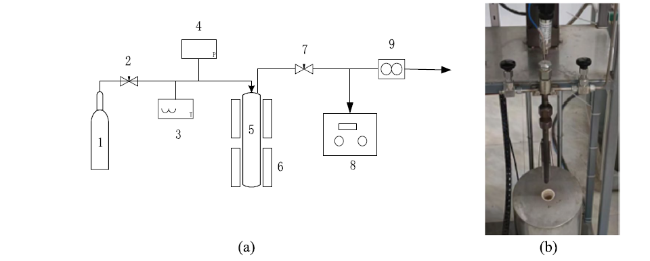
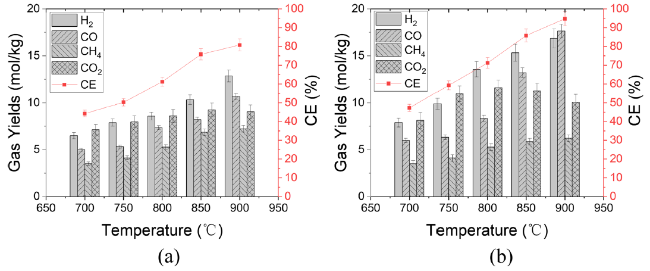
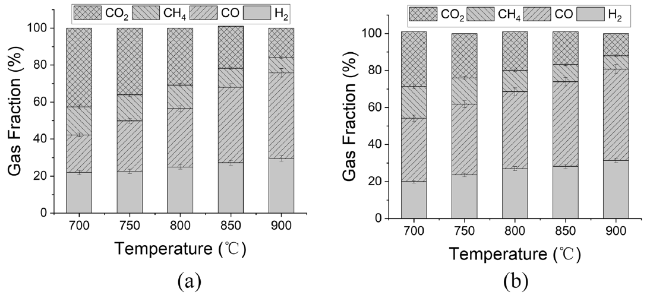
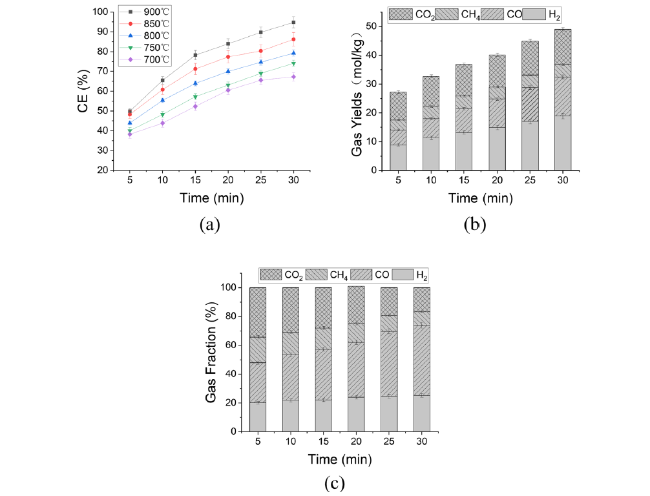
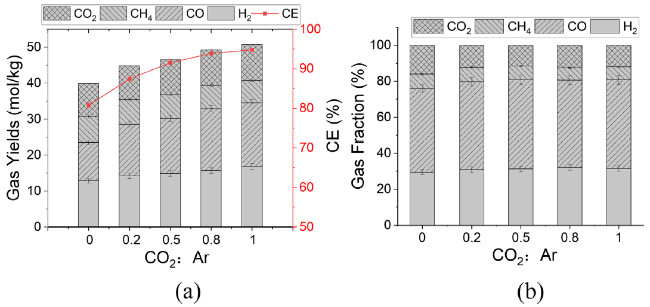
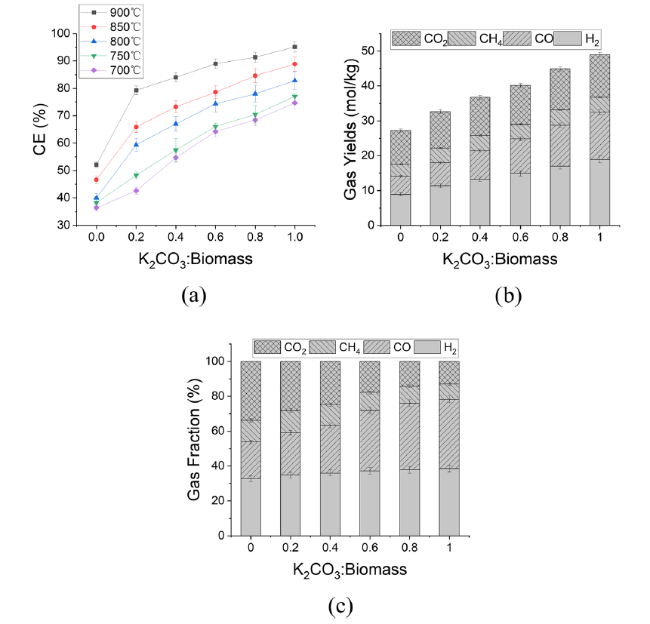
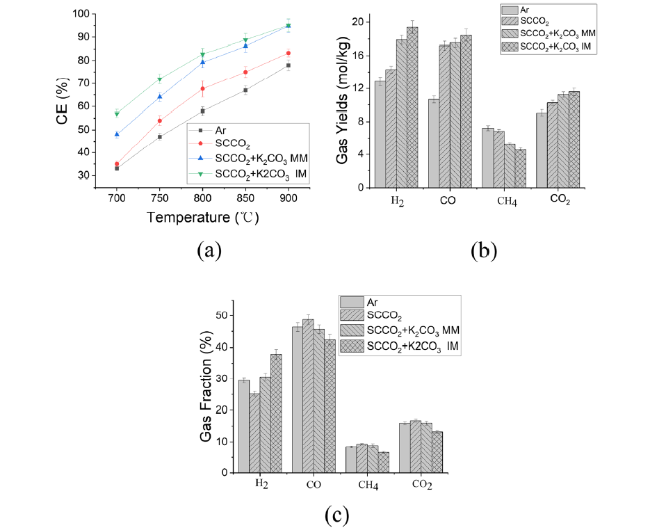
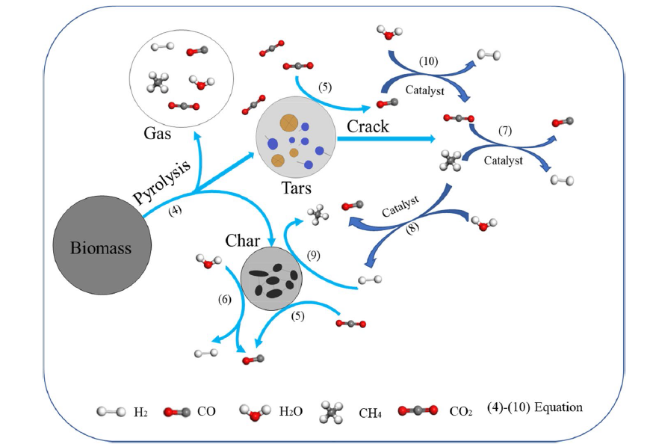
In addition to air and steam gasification of biomass, carbon dioxide gasification of biomass is currently the most widely concerned. The gasification of biomass carbon dioxide can further reduce the carbon dioxide in the environment, and can effectively reuse the carbon dioxide, again into carbon monoxide, for various ways of use. Mani et al. [
19] in their study of carbon dioxide gasification of wheat straw coke, pointed out that increasing the reaction temperature could effectively increase the conversion rate of the reaction. When the reaction temperature was 750 °C and the reaction time was 45 minutes, the conversion of straw coke could only reach 50%, while when the temperature was increased to 900 °C, the conversion of straw coke could reach 100% in the same reaction time. Seo et al. [
20] studied the carbon dioxide gasification of red pine coke at different temperatures and showed that an increase in reaction temperature increased the carbon monoxide content of the product, indicating that an increase in temperature facilitated the progress of the reaction. Huang et al. [
21] investigated the effect of metal catalysts on the rate of carbon dioxide gasification reaction of biomass coke, and the results showed that the rate of carbon dioxide gasification reaction increases with the increase of alkali metals, and different metal elements have different effects on the reaction. The catalytic efficiency of metal elements on the reaction was K > Na > Ca > Fe > Mg. Guizani et al. [
22] studied the effect of CO
2 introduction in a biomass fast gasification process at 850 °C. Because of reactions of CO
2 with gases, tars and char, the CO
2 and biochar yields decreased. Kwon et al. [
23] observed a substantial reduction in tar collection during the gasification of macroalgae in a 30% CO
2/70% N
2 atmosphere at 550 °C in the presence of CO
2. CO
2 enhances the gasification process by accelerating the thermal cracking of the volatile chemicals produced during the thermal degradation of the macroalgae. The gas yield is improved while the oil yield decreases. Gao et al. [
24] investigated the effect of CO
2 on the gasification process of lignite to varying degrees. They found that CO
2 promoted the gasification process of coal by enhancing the cleavage of benzene rings and the breakage of hydroxyl, methyl and methylene groups. The gas yield and surface area of the char were enhanced by further gasification with CO
2. When carbon dioxide and biomass are co-gasified, more carbon monoxide is produced to reduce carbon dioxide, effectively reducing carbon emissions and greenhouse gases, making more efficient use of biomass energy and meeting the needs of the current carbon neutral era [
25,
26,
27]. From the viewpoint of CO
2 consumption and CO yield enhancement, the utilization of CO
2 is preferable as a gasifying agent.