1 Introduction
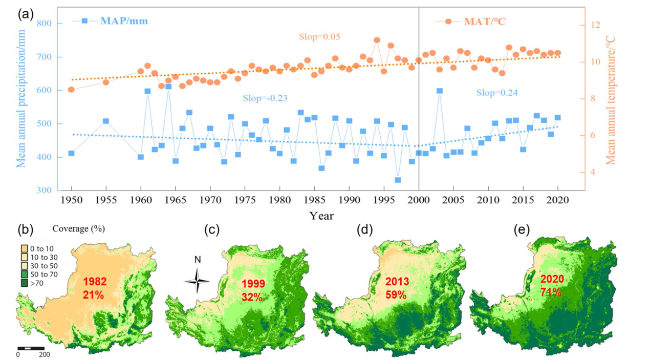
Fig. 2 Observed mean annual temperature (MAT) and precipitation (MAP) variations between 1950 and 2020 in the Loess Critical Zone (a). The slight orange dashed line (MAT) is fitted to the data with the slop = 0.05 (p < 0.001). The slight blue dashed line (MAP) is fitted to the data with the slop = -0.23 (before 2000) and slop = 0.24 (after 2000). Vegetation coverage from 1982 to 2020 in the Loess Critical Zone (b-e) |
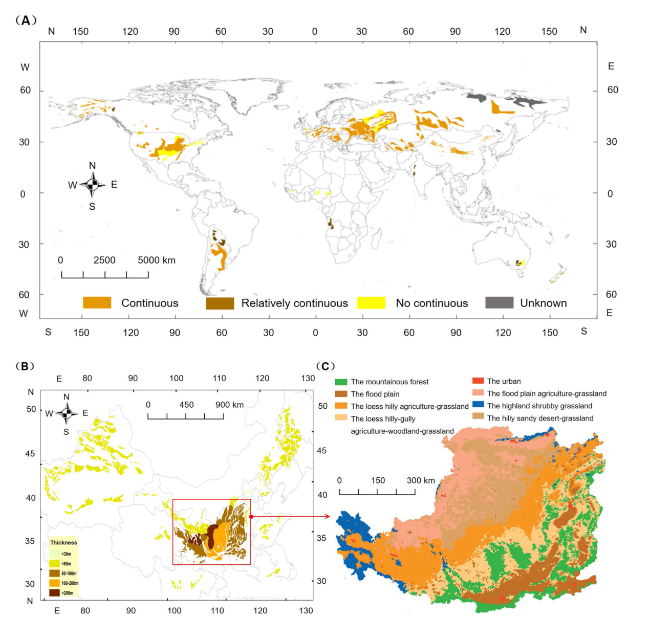
2 The development of the Loess Critical Zone
3 Distribution of the Loess Critical Zone
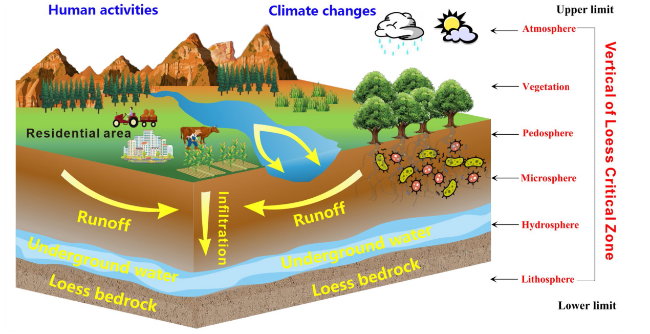
Fig. 3 The structural model of the Loess Critical Zone |
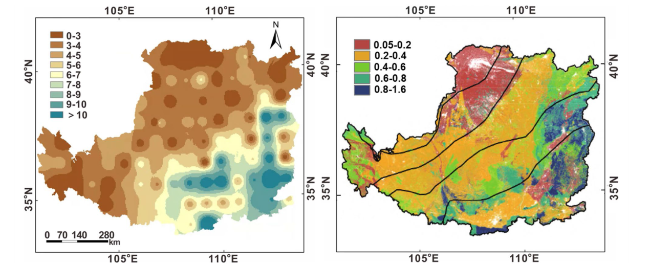
4 The stock and dynamic of SOC in the Loess Critical Zone
Table 1 Statistics of soil C density and stock in the Loess Plateau and China derived from various studies |
| Item | Soil depth (cm) | SOC density (kg m−2) | SOC stock (Pg) | Aboveground C stock (Pg) | Ecosystem C stock (Pg) | References |
|---|---|---|---|---|---|---|
| Loess Plateau | 0-20 | 0.76 | 0.85 | 1.51 | 2.84 | Yang et al., 2023 [26] |
| Loess Plateau | 0-20 | 0.67 | 1.52 | 0.44 | 2.29 | Li et al., 2021 [37] |
| Loess Plateau | - | - | - | - | 3.96 | Liu et al., 2018 [41] |
| Loess Plateau | 0-20 | 2.64 | 1.64 | - | - | Fu et al., 2014 [36] |
| Loess Plateau | 20-40 | 4.57 | 2.86 | - | - | Fu et al., 2014 [36] |
| Loess Plateau | 0-100 | 7.70 | 4.78 | - | - | Fu et al., 2014 [36] |
| Loess Plateau | 0-200 | 12.45 | 5.85 | - | - | Fu et al., 2014 [36] |
| Loess Plateau | 0-20 | 2.69 | 1.68 | - | - | Liu et al., 2011 [35] |
| Loess Plateau | 0-50 | 8.99 | 3.47 | - | - | Liu et al., 2011 [35] |
| Loess Plateau | 0-100 | 13.45 | 5.32 | - | - | Liu et al., 2011 [35] |
| Loess Plateau | 0-20 | 2.49 | 1.07 | - | - | Xu et al., 2003 [34] |
| China | 0-100 | 0.96 | 75.0 | 14.3 | 89.3 | Tang et al., 2018 [38] |
| China | 0-100 | 10.6-21.0 | 84.5 | 14.6 | 99.2 | Xu et al., 2003 [42] |
| China | 0-100 | 86.2 | 82.8 | 13.7 | 100.5 | Ji et al., 2008 [43] |
| China | 0-100 | 91.3 | 84.6 | 13.3 | 98 | Li et al., 2004 [44] |
| China | 0-10 | - | 69.4 | - | - | Xie et al., 2004 [45] |
| China | 0-100 | 125 | 119.8 | 35.2 | 155 | Ni, 2013 [46] |
| China | 0-100 | 4.86 | 50 | - | - | Pan et al., 2008 [47] |
| China | 0-100 | 10.83 | 100.2 | - | - | Wang et al., 2003 [48] |
| China | 0-100 | 102.9 | 100.6 | 52.5 | 153.1 | Peng and Apps, 1997 [49] |
| China | 0-100 | 20.3 | 185.7 | 6.1 | 191.7 | Fang et al., 1996 [50] |
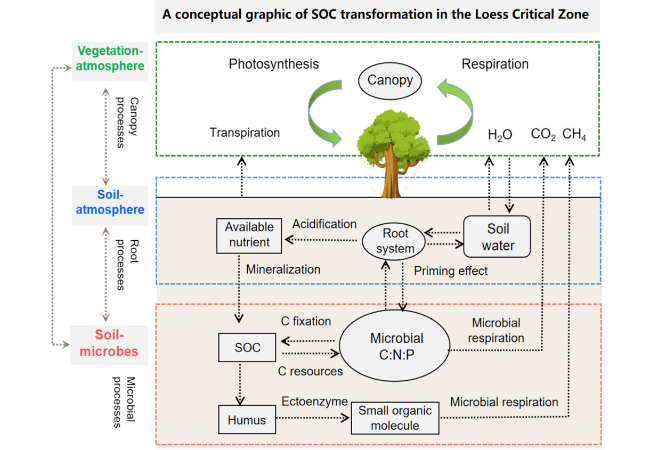
5 The microbial turnover of SOC in the Loess Critical Zone
Fig. 5 A conceptual graphic of SOC transformation in the Loess Critical Zone |
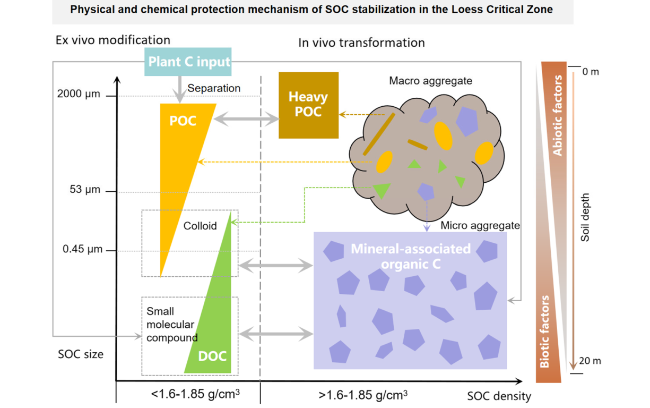
6 Mechanisms of SOC stabilization in the Loess Critical Zone
6.1 Physical and chemical protection of aggregates
Fig. 6 Physical and chemical protection mechanism of SOC stabilization in the Loess Critical Zone |
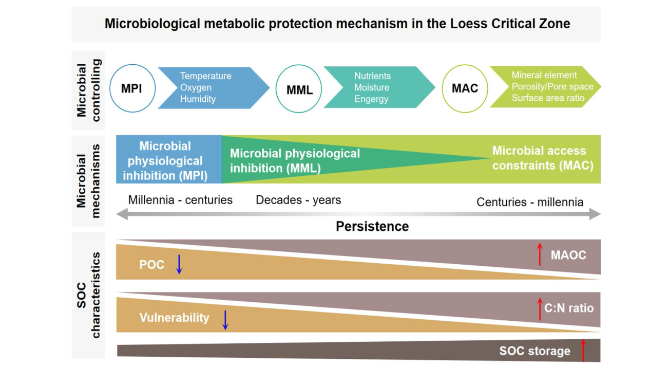
6.2 Microbiological metabolic protection
Fig. 7 Schematic representation of the mechanisms of stabilization of SOC in the Loess Critical Zone. Microbial physiological inhibition (MPI) is caused by freezing temperatures or lack of oxygen, and results in extremely low microbial processing of plant inputs, which thus accumulate largely as particulate organic C (POC) and can persist for millennia, forming C-rich organic soils characterized by low C:N ratios. MPI transitions to microbial metabolic limitation (MML) when conditions still limit but do not entirely inhibit microbial activity. Thus, MML is controlled by the availability of energy, nutrients, and moisture to microorganisms. High levels of MML result in higher accumulation of soil organic matter (SOC) in POC, higher soil C:N, relatively low SOC persistence, and high SOC vulnerability to changes that may release MML. Microbial access constraints (MAC) limit the access of microorganisms and their enzymes to SOC. Since organo-mineral associations and protection within small pores are the main spatial constraints on microbial access to SOC, MAC is controlled by the soil mineral capacity to form strong organo-mineral bonds (i.e., Al, Fe, Ca, and available reactive surface area), and by pore space and moisture. Most of the SOC protected by MAC is in MAOC, which may persist in soils for up to millennia and is generally less vulnerable to environmental changes. In soils with lower overall SOC stocks, where available SOC is likely have undergone microbial processing, MAC is the prevailing mechanism of SOC persistence |
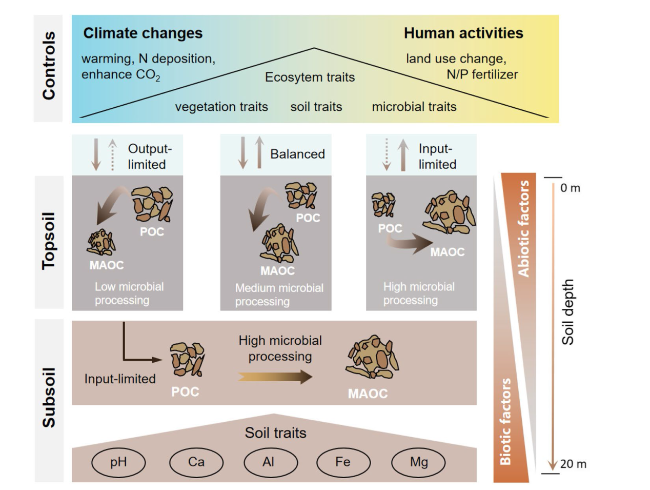
7 Controls of SOC persistence in the Loess Critical Zone
7.1 Climate change
Fig. 8 Conceptual representation of SOC persistence framework in the Loess Critical Zone. Arrows in top and bottom panel are color-coded according to potential controls. We hypothesize (right panel) that C input limits soil C cycling when plant photosynthesis is constrained relatively more than microbial activities. In these ecosystems, microorganisms process the small available plant C inputs, resulting in low soil C stocks with relatively high mineral-associated organic C (MAOC) accumulation controlled by the availability of soil minerals for stabilization of microbial products. C input-limited ecosystems are expected to have decoupled C cycling. We also hypothesize (left panel) C output limitation to control soil C cycling in systems where microorganisms are more inhibited than plants. C-output limited systems would be characterized by higher POC relative to MAOC. Further, we hypothesize (central panel) that balanced plant C inputs vs microbial C outputs will result in soils having more equal shares of POC and MAOC. Further, we expect climate changes and human activities to be the main driver of C input- and C output-limited systems, while ecosystem traits emerging from the interaction of plant, microbial, and soil traits to be significant drivers of soil C dynamics in ecosystems with more balanced inputs and outputs. Finally, we hypothesize (bottom panel) that subsoil are input-limited and their SOC dynamics are largely controlled by soil traits, including mineral properties |
7.2 Biotic and abiotic factors
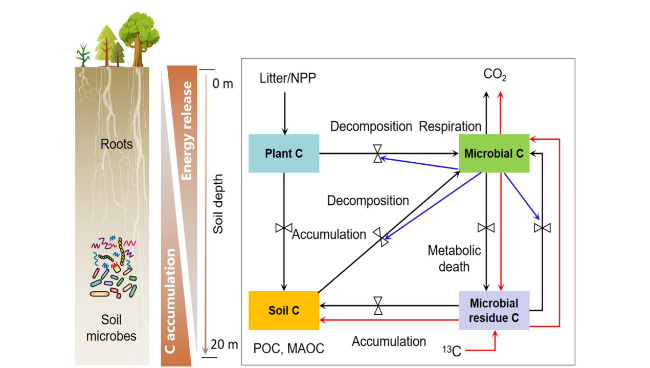
8 Soil C cycling model in the Loess Critical Zone
Fig. 9 Model of C pools model of SOC cycling in the Loess Critical Zone (including microbial biomass poll and residue C pool). Note: There are two pathways through which soil organic carbon is formed from plant litter. The first pathway involves the direct sedimentation and coagulation of litter C modified by microbial extracellular enzymes into the soil. The second pathway is an indirect transformation where litter carbon is first assimilated by soil microorganisms and then synthesized into microbial biomass C. When these microorganisms die, their residues accumulate in the soil, contributing to the organic carbon pool. The direction and magnitude of these processes are influenced by environmental factors, as indicated by the valve symbols on each arrow. The blue solid line represents the Michaelis-Menten kinetics, which regulates the size and dynamics of the microbial biomass C pool. The red solid line arrow represents the pathway when.13C-labeled C in microbial residues is introduced into the system (adapted from [74]) |