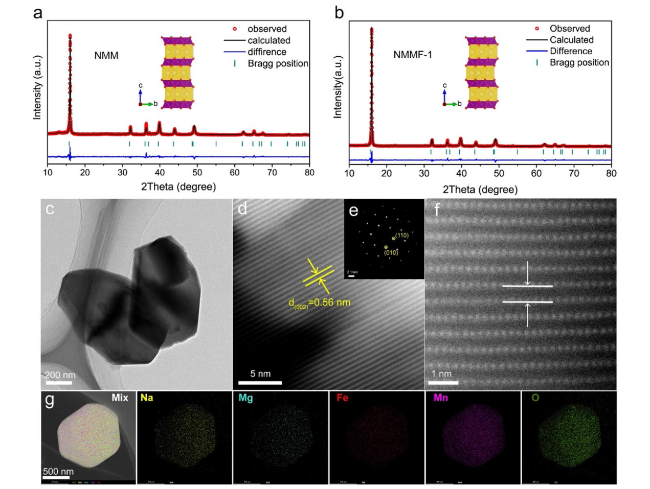
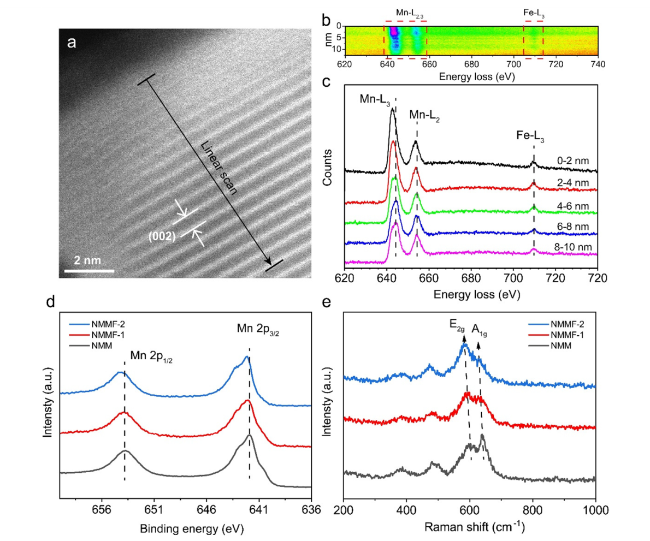
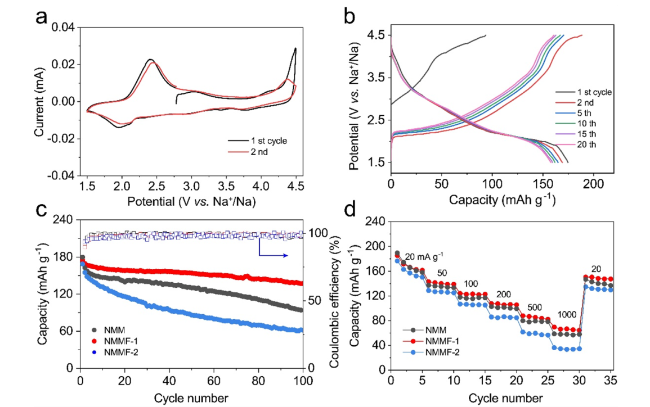
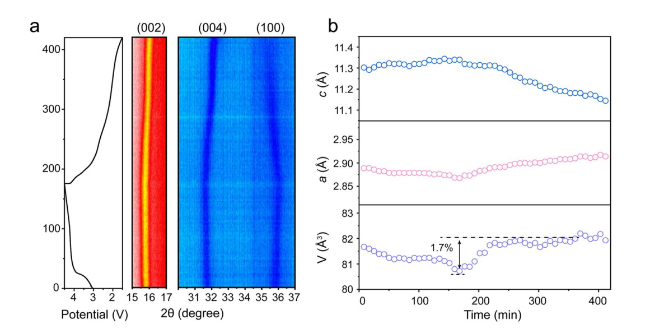
Layered transition metal oxides (Na
xTMO
2, TM = transition metal) can be categorized into two main groups (P2- and O3-types) according to the sodium ion accommodating sites and the oxygen stacking sequences [
19]. Compared with the O3-type Na
xTMO
2, the low-Na P2-type materials have many vacancies in Na layers and possess wide prismatic paths for sodium ion diffusion with a low diffusion barrier, which makes P2-type Na
xTMO
2 a promising cathode material [
20,
21,
22]. However, most P2-type Na
xTMO
2 are generally stable in the potential range of 2-4.1 V (
vs. Na
+/Na) with low discharge capacities. With the widening of voltage range, the P2-type Na
xTMO
2 is susceptible to phase transition (P2-O2/PO4) or irreversible oxygen redox, leading to drastic volume change and poor structural stability [
23,
24,
25]. In addition, the existence of Mn
3+ would present Jahn-Teller distortion, which lowers the Na
+ mobility [
26,
27,
28]. Although inactive element doping (Mg
2+ [
29,
30], Al
3+ [
31,
32], Li
+ [
33], Zn
2+ [
34], and Ti
4+ [
35,
36] etc.) have been proved to have a significant improvement in the structural stability of Na
xTMO
2. However, the inactive elements can't provide extra charge compensation, which decreases the theoretical capacities. Therefore, when considering the synthetic effect of introducing extra redoxes and stabilizing structure, the active elemental doping is an effect approach. For example, Wang et al. reported that the strong-electronegativity Cu
2+/Cu
3+ redox can stabilize the Na-deficient P2-Na
2/3Mn
0.72Cu
0.22Mg
0.06O
2 phase to achieve reversible cationic and anionic redoxes [
37]. Myung et al. developed the active Ni
2+ doped P2-Na
0.75[Li
0.15Ni
0.15Mn
0.7]O
2 cathode material, and the active Ni doping not only decreases the voltage hysteresis but also improves the cycling stability [
38]. From this point, searching for earth-abundant and moderate active element doping is urgently required.