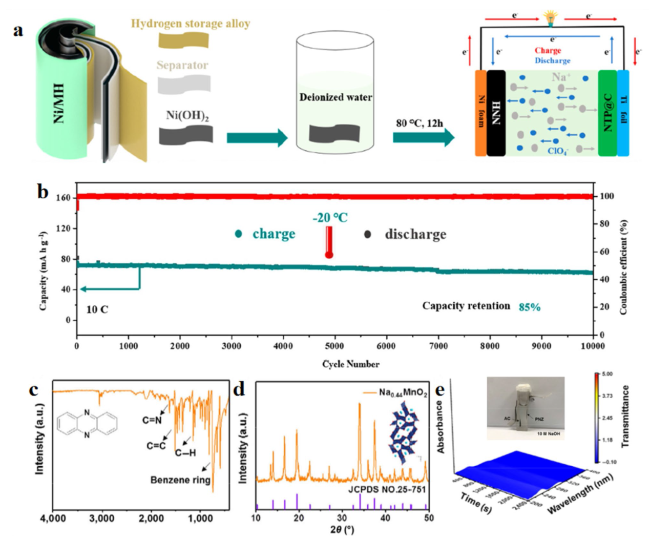
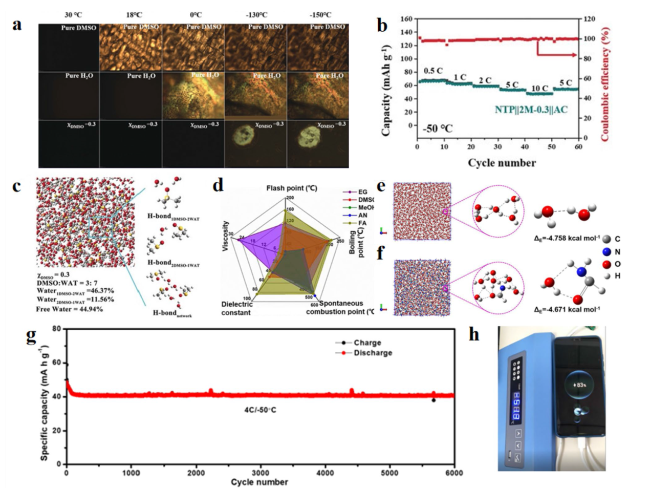
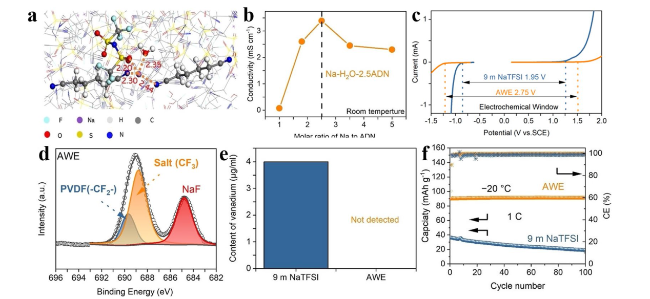
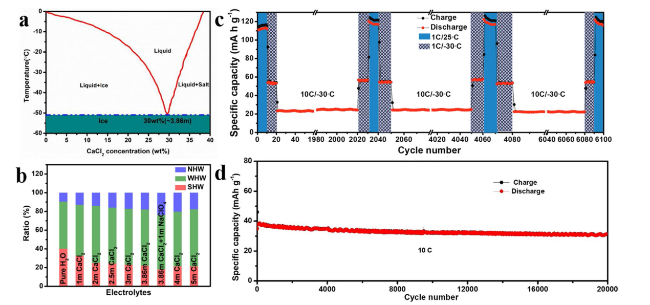
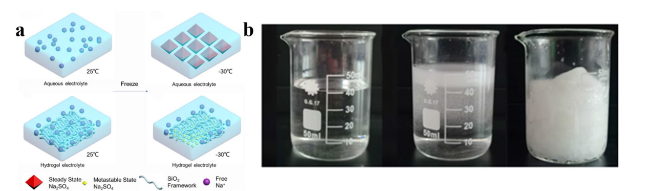
To cope with extreme conditions while fulfilling safety and environmental requirements, extensive systematic research is necessary for subzero-temperature aqueous sodium-ion batteries (SASIBs) [
22]. Several challenges currently exist in SASIBs research: (1) Narrow electrochemical stability window: due to the thermodynamic and kinetic properties of water, the ESW of water is relatively narrow, only 1.23 V, which limits the voltage range and thus affects the energy density of SASIBs. If the voltage exceeds this range during the charging process, the electrolytes may decompose, leading to a decreased performance of SASIBs. Furthermore, severe hydrogen evolution and oxygen evolution reactions occur on the electrode surface, leading to surface corrosion and structural damage of the electrode materials, ultimately reducing the lifespan of SASIBs. Due to the limited ESW, there are relatively few electrode materials that can function stably within this window, which restricts the choice of electrode materials. Finding new electrode materials may be necessary to meet practical needs [
23,
24]. (2) Electrolyte solidification and Low ionic conductivity: when the temperature approaches the freezing points, water molecules bind together through strong hydrogen bonds, reducing fluidity, enhancing electrolytes viscosity, and hindering the mass transfer process [
25]. The resulting frozen electrolytes are incapable of efficiently transporting ions and reducing the diffusion rate of ions. This causes poor contact between the electrolyte and electrode interface, preventing normal reactions on the electrode surface, increasing the concentration polarization, and decreasing the ionic conductivity of the SASIBs [
24]. (3) Salt precipitation: the solubility of sodium salts can decrease due to the aggregation and increased order of solvent molecules at low temperatures. This can lead to their deposition and potentially cause corrosion of certain components in the battery, resulting in damage to its structure and performance, ultimately leading to short circuits or failure [
26]. (4) Dendrite growth: in a low-temperature environment, the movement speed of ions slows down and the efficiency of charge transfer decreases. The transport rate of sodium ions in an aqueous electrolyte is faster than their embedding in the anode electrode materials, leading to the accumulation of a significant amount of Na
+ deposited on the surface of the anode. The uneven deposition of sodium can form sodium dendrites that may puncture the separators, causing a short circuit in SASIBs and compromising their safety performance [
23,
27]. Improving the output voltage, energy density, and cycle life of SASIBs is crucial. Numerous studies have aimed to achieve this by regulating the interfacial electrochemical reaction process of aqueous electrolytes, inhibiting side reactions at the electrode interface, and broadening its ESW.