1 Introduction
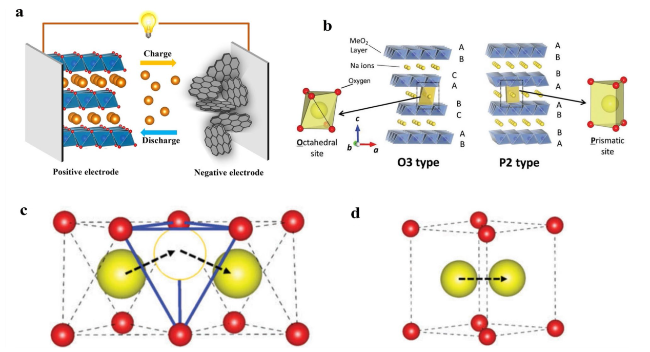
Fig. 1 a Working principles diagram of sodium ion batteries. b Schematic illustrations of crystal structures of O3 and P2 type NaxTMO2. Reproduced with permission [46]. Copyright 2014, American Chemical Society. c The indirect Na-ion diffusion path with the intermediate tetrahedron site in O-type frameworks. d The direct Na-ion diffusion path without the intermediate site in P-type frameworks. The red and yellow balls represent oxygen ions and sodium ions, respectively. Reproduced with permission [47]. Copyright 2016, Royal Society of Chemistry |
Table 1 Summary of the electrochemical performance of recent reported O3-type layered cathode materials for SIBs |
| Materials | Voltage windows(V) | Reversible capacity(mAh g−1) | Capacity retention | Ref |
|---|---|---|---|---|
| NaNi0.45Cu0.05Mn0.4Ti0.1O2 | 2-4 | 124 | 70.2% (500 cycles at 1 C) | [11] |
| NaFeO2 | 2.5-3.4 | 80 | 75% (30 cycles at 0.1 C) | [48] |
| Na4FeRuO6 | 2-4 | 120 | 80% (100 cycles at 2 C) | [49] |
| NaFeO2@C | 2-3.4 | 89.6 | 83.7% (100 cycles at 0.1 C) | [50] |
| NaMnO2 | 2-3.8 | 185 | 71.4% (20 cycles at 0.1 C) | [51] |
| NaMn0.6Al0.4O2 | 1-4 | 160 | 81% (100 cycles at 500 mA g−1) | [52] |
| Na1.2Mn0.4Ir0.4O2 | 1.5-4.4 | 179 | 60% (50 cycles at 0.5 C) | [53] |
| Na[Li0.05Mn0.50Ni0.30Cu0.10Mg0.05]O2 | 2-4 | 172 | 70.4% (1000 cycles at 20 C) | [54] |
| NaNiO2 | 1.25-3.75 | 123 | 94.3% (20 cycles at 0.1 C) | [55] |
| Na0.8Ni0.6Sb0.4O2 | 2-4.3 | 106 | 78% (100 cycles at 1 C) | [56] |
| Na0.7Ni0.35Sn0.65O2 | 2-4 | 64 | 80% (100 cycles at 0.1 C) | [57] |
| NaTi0.5Ni0.5O2 | 2-4 | 102 | 75% (300 cycles at 1 C) | [58] |
| Na0.8Ni0.3Co0.1Ti0.6O2 | 2-4 | 92 | 98% (1000 cycles at 5 C) | [59] |
| Na0.9Ni0.3Co0.15Mn0.05Ti0.5O2 | 2-4 | 112.7 | 81.6% (1000 cycles at 100 C) | [60] |
| Na3Ni2RuO6 | 2-3.8 | 130 | 81% (1000 cycles at 1 C) | [61] |
| Na3Ni2SbO6 | 2-4 | 120 | 70% (500 cycles at 2 C) | [62] |
| NaNi0.5Mn0.2Ti0.3O2 | 2-4 | 135 | 85% (200 cycles at 5C) | [63] |
| NaNi0.47Zn0.03Mn0.5O2 | 2-4 | 113 | 80% (150 cycles at 0.5C) | [64] |
| NaNi0.5Mn0.1Sn0.4O2 | 2-4.2 | 91 | 85% (200 cycles at 0.1 C) | [65] |
| MgO-NaNi0.5Mn0.5O2 | 2-4.2 | 167 | 75% (100 cycles at 0.5 C) | [66] |
| NaFe0.2Ni0.4Mn0.4O2 | 2-4 | 131 | 96.4% (30 cycles at 0.05 C) | [67] |
| NaFe0.3Ni0.35Mn0.35O2 | 2-4 | 130.3 | 80% (100 cycles at 1 C) | [68] |
| NaNi0.4Fe0.2Mn0.2Ti0.2O2 | 2-4.2 | 145 | 84% (200 cycles at 0.1 C) | [69] |
| Na0.85Li0.1Ni0.175Mn0.525Fe0.2O2 | 2-4.5 | 160 | 88% (100 cycles at 1 C) | [70] |
| NaNi1/3Fe1/3Mn1/3O1.99F0.01 | 2-4 | 122 | 85% (70 cycles at 1 C) | [71] |
| NaNi0.25Mg0.05Cu0.1Fe0.2Mn0.2Ti0.1Sn0.1O2 | 2-4 | 130.8 | 75% (500 cycles at 1 C) | [72] |
| Na0.94Ni0.29Cu0.1Fe0.16Mn0.3Ti0.15O2 | 2-4 | 122 | 79% (300 cycles at 0.5 C) | [73] |
| Na2SiO3@NaNi1/3Fe1/3Mn1/3O2 | 1.5-4.2 | 134 | 83.3% (200 cycles at 0.5 C) | [74] |
| ZrO2@NaNi1/3Fe1/3Mn1/3O2 | 1.5-4.3 | 152.4 | 81.9% (100 cycles at 1 C) | [75] |
| Na7/9Ni2/9Mn4/9Fe1/9Mg1/9Li1/9O2 | 2.0-4.4 | 170.5 | 71.8% (500 cycles at 5 C) | [76] |
1.1 O3-type layered structure
1.1.1 O3-type NaxFeO2
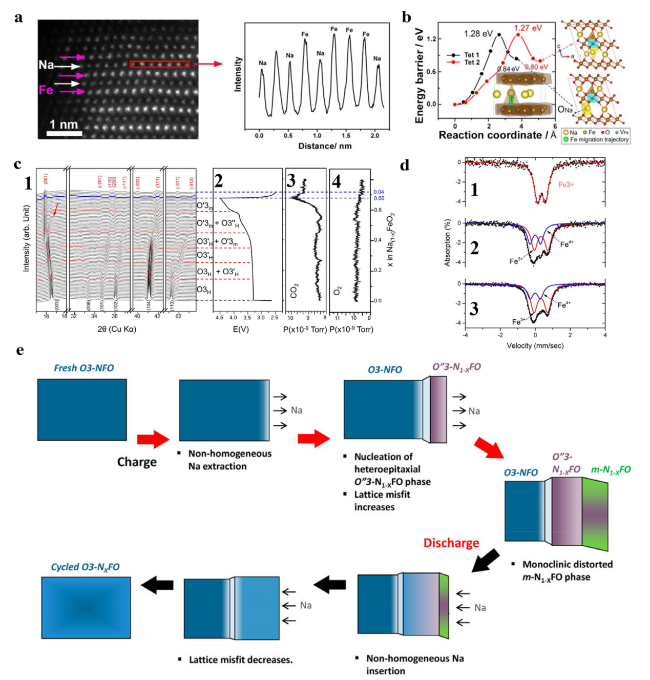
Fig. 2 a The HAADF images of NaFeO2 at the fully charged state and the corresponding line profile acquired from the red rectangular region. b Pathways for the direct Fe migration from the OFe to the TNa sites in Na0.33FeO2. The yellow, brown, red, blue and green balls in the structural schematic are for Na, Fe, O, VFe and the Fe migration trajectory, respectively. Reproduced with permission [77]. Copyright 2018, Elsevier B.V. c c1 In-situ synchrotron X-ray diffraction patterns of Na1−xFeO2 showing the characteristic hkl peak in different phases. c2 In-situ galvanostatic charge and discharge profiles at a rate of 0.05 C with a cutoff voltage of 4.5 V. The arrow indicates broadening of the (003) peak of O3’H. c3 and c4 CO2 and O2 gas evolution from NaFeO2 during sodium extraction and insertion. Reproduced with permission [78]. Copyright 2019, American Chemical Society. d Ex situ Mössbauer spectra for NaFeO2 electrodes a1 in the pristine state, a2 charged to 0.46 SOC, and a3 stored for 2 days under open circuit conditions after being charged to 0.5 SOC. e Schematic illustration of the proposed coherent phase evolution during electrochemical cycling of α-NaFeO2. Reproduced with permission [79]. Copyright 2015, American Chemical Society |
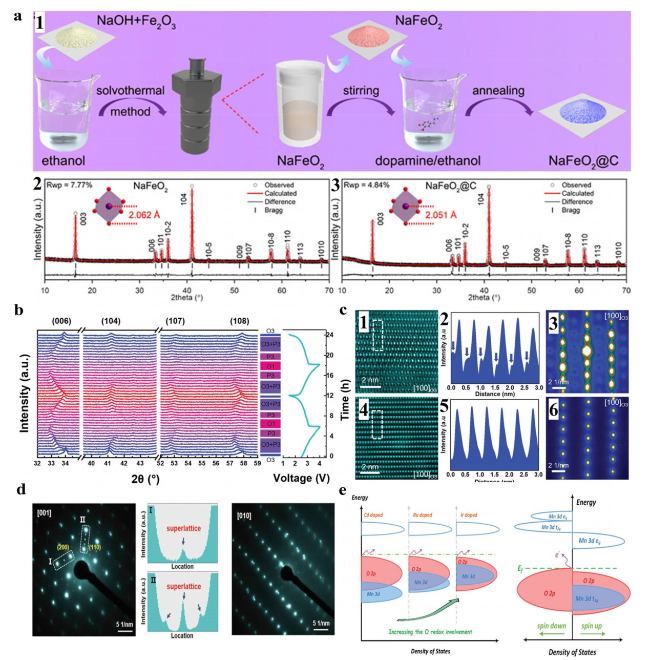
Fig. 3 a a1 Schematic illustration of the synthesis procedure of the NaFeO2 and NaFeO2@C samples. Rietveld-refined XRD patterns of the NaFeO2 a2 and NaFeO2@C a3 samples, respectively. Reproduced with permission [50]. Copyright 2021, Elsevier B.V. b In situ XRD patterns of the NFRO cathode under a current rate of 0.25C at voltage range between 1.5 and 4 V. c Atomic STEM-HAADF images of c1 NFO and c4 NFRO samples charging to 4 V; c2 Line-profile of selected area by dash line in c1, the blue arrow in c2 indicates the migration of Fe ions into Na layers; c5 Line-profile of selected area by dash line in c4; Nanobeam electron diffraction patterns of c3 NFO and c6 NFRO samples charging to 4 V. Reproduced with permission [49]. Copyright 2019, Wiley-VCH. d SAED patterns of sample viewed along the [1] axis and [10] axis, respectively, (I and II are the corresponding line-profiles of the marked regions in SAED pattern). Reproduced with permission [52]. Copyright 2021, Wiley-VCH. e Schematic illustration of the DOS for chosen potential doping species and Ir-doped Na1.2Mn0.4Ir0.4O2. The O-2p band and Mn 3d band are depicted in red and blue, respectively. The Cd Ru and Ir doped Mn-based Na-rich system has a tendency for increasing O redox improvement as the Mn 3d band increases from the deep part, which refers to a suppression of O2 release. Reproduced with permission [53]. Copyright 2019, Wiley-VCH |
1.1.2 O3-type NaxMnO2
1.1.3 O3-type NaxNiO2
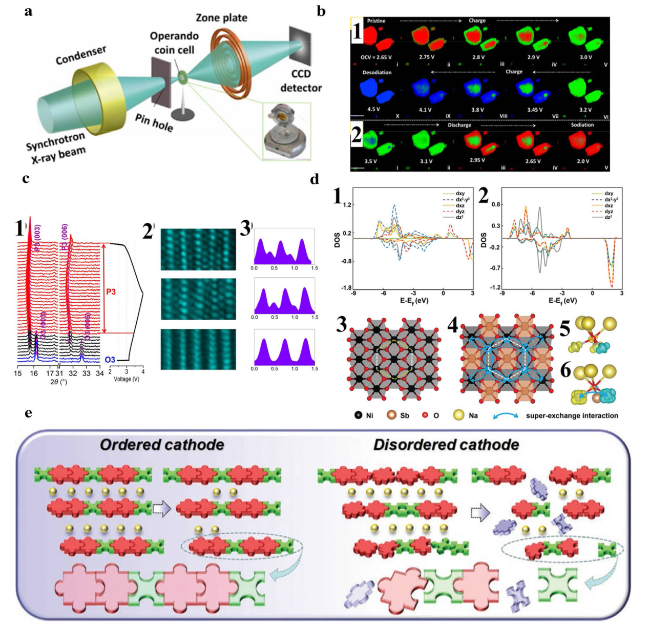
Fig. 4 a Schematic illustration of the TXM experimental setup. b b1 and b2 are the two-dimensional chemical phase mapping during the first charge and discharge process. Scale bar: 10 µm. Reproduced with permission [107]. Copyright 2017, Elsevier B.V. c c1 In-situ XRD patterns collected for NNCT electrode cycled in the voltage range of 2.0-4.0 V. The O3-type phase, biphase coexistence, and P3-type phase are represented by blue, black, and red colors, respectively. c2 STEM images of the pristine (bottom), fully charged (middle), and fully discharged (top) NNCT electrodes viewed along the [0 1 0] direction. c3 The corresponding line profiles of the STEM images. Reproduced with permission [59]. Copyright 2018, Elsevier B.V. d d1 and d2 The partial density of states (pDOS) of NaNiO2 and NaNi2/3Sb1/3O2. d3 and d4 The comparison of bond length of NaNiO2 and NaNi2/3Sb1/3O2 structure. d5 and d6 The spin difference charge density of NaNiO2 and NaNi2/3Sb1/3O2. Reproduced with permission [117]. Copyright 2019, Wiley-VCH. e Schematic diagram showing that the ordered arrangement of transition metal (TM) in layered oxide could provide a more stable structure for long-term (de)intercalations of Na + in comparison with that of the disordered arrangement. Reproduced with permission [61]. Copyright 2020, Wiley-VCH |
1.1.4 O3-type NaxNiyMn1-yO2
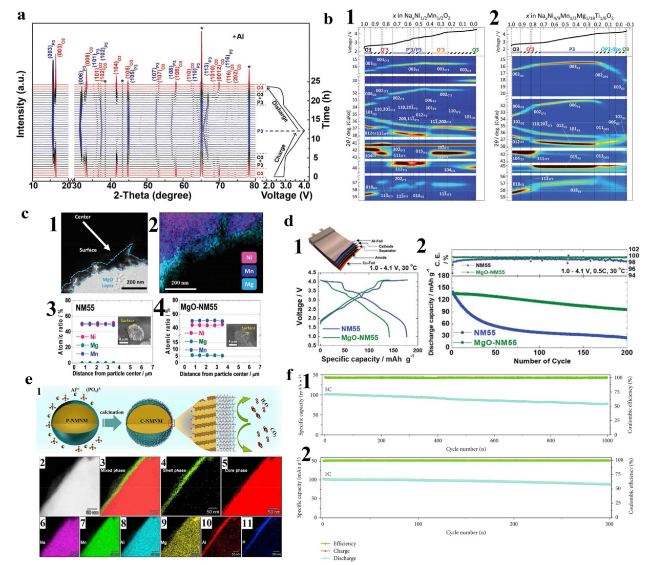
Fig. 5 a In situ XRD patterns collected during the first charge/discharge of the Na/NaNi0.5Mn0.2Ti0.3O2 cell under a current rate of 0.05 C at voltage range between 2 and 4 V. Black asterisks represent peaks from Al window. Reproduced with permission [63]. Copyright 2017, Wiley-VCH. b Contour plots of operando XRD patterns for b1 Non-sub and b2 Mg-Ti-sub electrodes during 1st charging to 4.5 V. Reproduced with permission [127]. Copyright 2021, Royal Society of Chemistry. c c1 Bright field TEM image and c2 corresponding EDS mapping of MgO-NM55. EPMA results of c3 NM55 and c4 MgO-NM55 cathodes. Inset images display the cross-sectional SEM images of NM55 and MgO-NM55 particles, respectively. d Electrochemical performances of pouch-type NM55/hard carbon and MgO-NM55/hard carbon full cells: d1 initial charge-discharge voltage profiles at 15 mA g−1 and d2 coulombic efficiency and long-term cycling stability test at 0.5 C-rate (75 mA g−1). Reproduced with permission [66]. Copyright 2018, Royal Society of Chemistry. e e1 Schematic illustration of the synthetic process for the AlPO4 coating on the surface of Na[Li0.05Mn0.50Ni0.30Cu0.10Mg0.05]O2. e2 High-angle annular dark field (HAADF)-STEM image. STEM-EDS mapping of crystalline phase: Mixed phase of C-NMNM e3, AlPO4 shell phase e4 and core phase of Na[Li0.05Mn0.50Ni0.30Cu0.10Mg0.05]O2 (e5). (e6-e11) Element Na (e6), Mn (e7), Ni (e8), Mg (e9), Al (e10) and P (e11) distributions from the edge to the bulk material of C-NMNM. Reproduced with permission [129]. Copyright 2019, Cell Press. f f1 Cycling performance during 1000 cycles at 5C after performance tests at various rates. f2 Electrochemical performance of full-cell system Cycling performance during 300 cycles at 1C. Reproduced with permission [134]. Copyright 2020, American Association for the Advancement of Science |
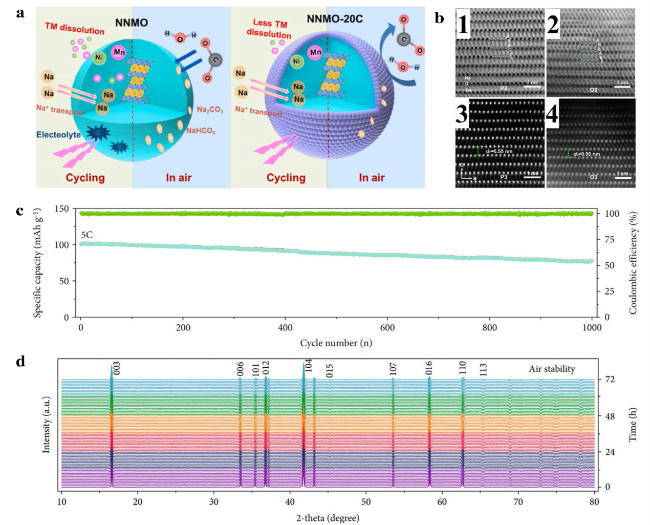
Fig. 6 a Schematic illustration of the advantages for the Al2O3-surface modification in stabilizing the surface and bulk structure of the NNMO cathode. Reproduced with permission [133]. Copyright 2019, Elsevier B.V. b ABF-STEM images of water-soaked (b1) NaNM and (b2) NaNCMT. HAADF-STEM images ofwater-soaked (b3) NaNM and (b4) NaNCMT. Reproduced with permission [11]. Copyright 2018, American Chemical Society. c Cycling performance during 1000 cycles at 5C after performance tests at various rates. d In situ XRD patterns of air-exposure stability test for three days (the different colour regions stand for the different air-exposure stages). Reproduced with permission [134]. Copyright 2020, AAAS |
1.1.5 O3-type NaxNiyMnzFe1-y-zO2
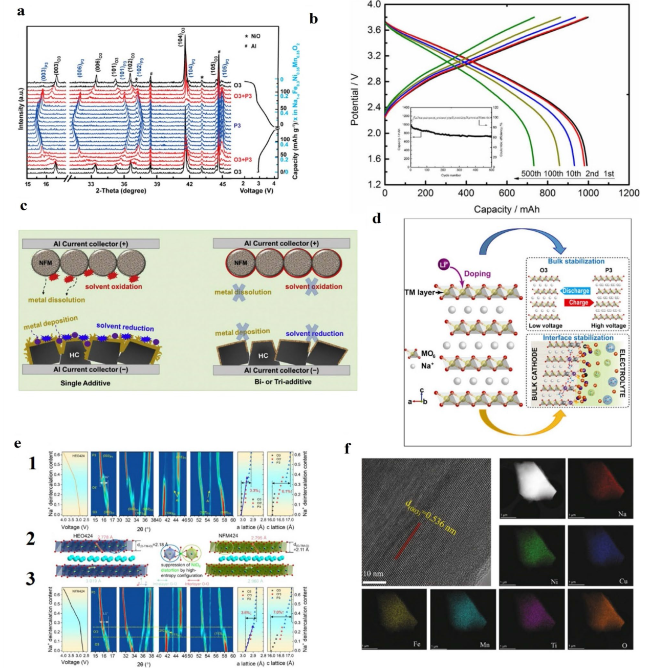
Fig. 7 a In situ XRD patterns of NaFe0.3Ni0.35Mn0.35O2 tested within 2.0 − 4.0 V, in which the black and blue scales (right) represent capacity and corresponding x value in Na1−xFe0.3Ni0.35Mn0.35O2, respectively. Reproduced with permission [68]. Copyright 2019, American Chemical Society. b The voltage profiles of a 1 Ah soft-packed sodium ion battery cycled between 1.5 V and 3.8 V at 1 C rate. Reproduced with permission [27]. Copyright 2019, Electrochemical Society, Inc. c Schematic summary on the role of PST and DTD additives in HC/NFM full cell. Reproduced with permission. [28] Copyright 2018, Elsevier B.V. d Dual-stabilization effect of the cation dopants on the evolution of bulk structure and electrode-electrolyte interphase of a Li-substituted O3-type cathode. Reproduced with permission [70]. Copyright 2018, Cell Press. e Structural evolution during the initial charge process. 2D contour plots of in situ XRD during the structural evolution of HEO424 e1 and NFM424 e3 cathodes. e2 Schematic illustrations showing the TMO2 slabs in both cathodes. The first charge curves as a function of Na+ deintercalation content are plotted on the right, and the a/c-axis lattice parameter changes in the as-prepared samples obtained by fitting the in situ XRD data are plotted on the left. Reproduced with permission [72]. Copyright 2022, American Chemical Society. f HRTEM image and EDS mapping of NNCFMTO sample. Reproduced with permission [73]. Copyright 2021, Elsevier B.V |
1.1.6 Ion doping
1.1.7 Surface modification
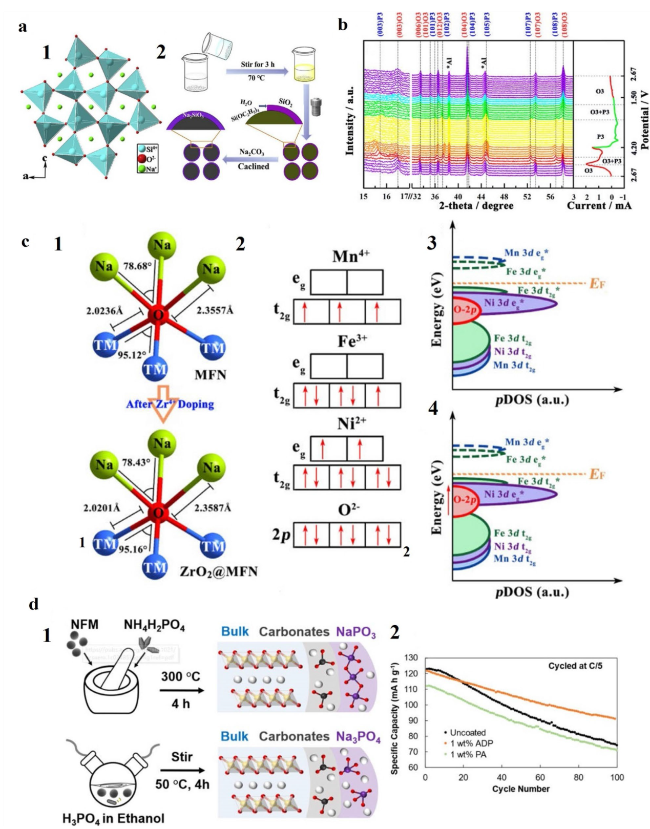
Fig. 8 a a1 The structure of Na2SiO3. a2 Schematic illustration of the synthesis process for Na2SiO3@NaNi1/3Mn1/3Fe1/3O2. Reproduced with permission [74]. Copyright 2019, Elsevier B.V. b In situ XRD patterns of TiO2@MFN during the charge − discharge process. Reproduced with permission [36]. Copyright 2020, American Chemical Society. c c1 Na-O-TM structure before and after Zr4+ doping. c2 Mn4+, Fe3+, Ni2+ and O2− electronic states of the outermost orbit. DOS schematic diagrams of c3 MFN and c4 ZrO2@MFN. Reproduced with permission [75]. Copyright 2021, Elsevier B.V. d d1 Illustrated diagram of the ammonium dihydrogen phosphate (top) and phosphoric acid (bottom) coating methods. d2 Cycling performance of uncoated and coated NFM at a C/5 rate. Reproduced with permission [100]. Copyright 2021, American Chemical Society |
1.2 Composite structure construction
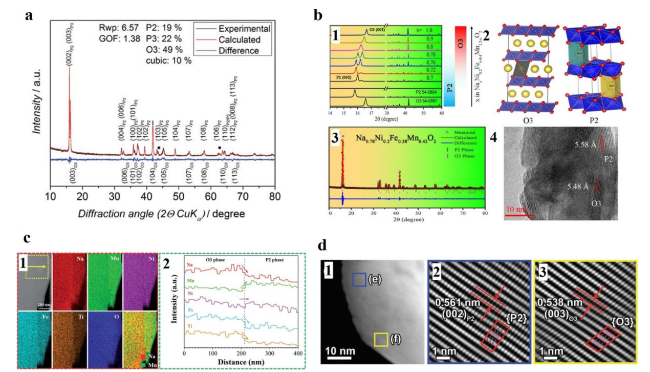
Fig. 9 a X-ray diffraction pattern and Rietveld refinement of P3/P2/O3-material (cubic Mg1-xNixO (x≈0.3) phase is marked with squares). Reproduced with permission [154]. Copyright 2016, Wiley-VCH. b b1 X-ray diffraction patterns of NaxNi0.2Fex-0.4Mn1.2-xO2 with different Na contents (0.7 − 1.0) and the phase evolution derived from XRD patterns is shown in the right. b2 Schematic illustration of O3 (left) and P2 (right) structures. b3 Observed and calculated XRD profiles for NNFM-0.78 with b4 TEM image of this material. Reproduced with permission [40]. Copyright 2017, American Chemical Society. c c1 STEM-EDX HAADF image and elemental distribution maps; c2 line profile representing the elemental intensities averaged over 360 nm parallel to the interface. Reproduced with permission [155]. Copyright 2021, Royal Society of Chemistry. d d1-d3 HRTEM images of the biphasic domain of NMFML. Reproduced with permission [76]. Copyright 2022, Wiley-VCH |