1 Introduction
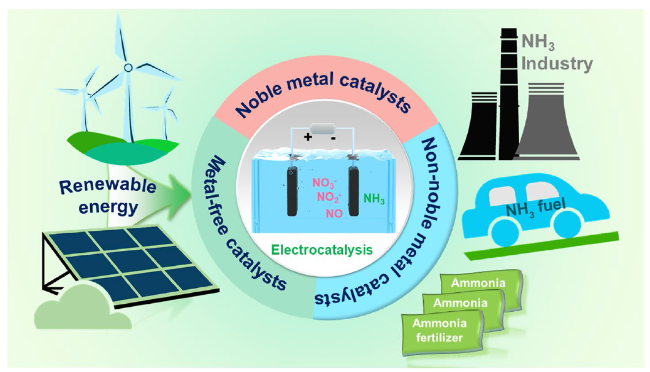
Fig. 1 The schematic illustration of ammonia production from nitrogenous waste through electrocatalytic reduction driven by renewable energy |
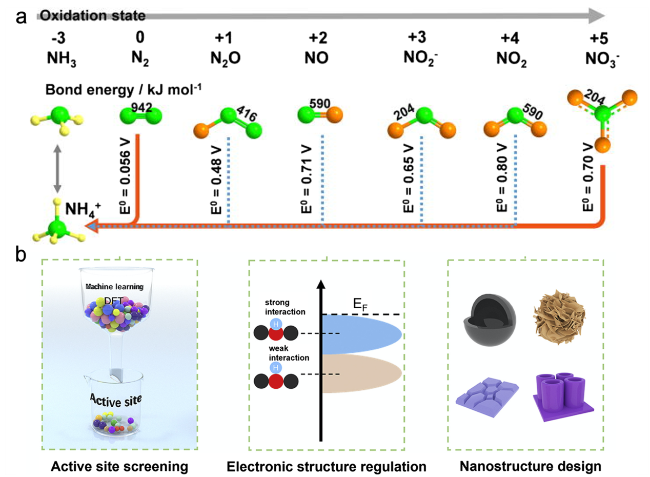
2 Reaction mechanism
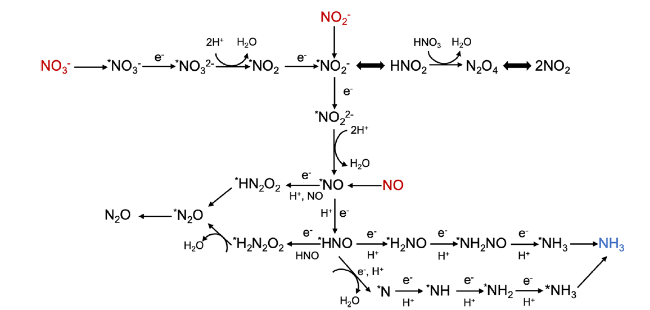
2.1 Reaction pathways
Fig. 2 Reaction pathways diagram of several nitrogenous species (NO3-, NO2-, and NO) in the aqueous solution |
2.2 Competition with hydrogen evolution reaction
3 Electrocatalysts for the NO3RR
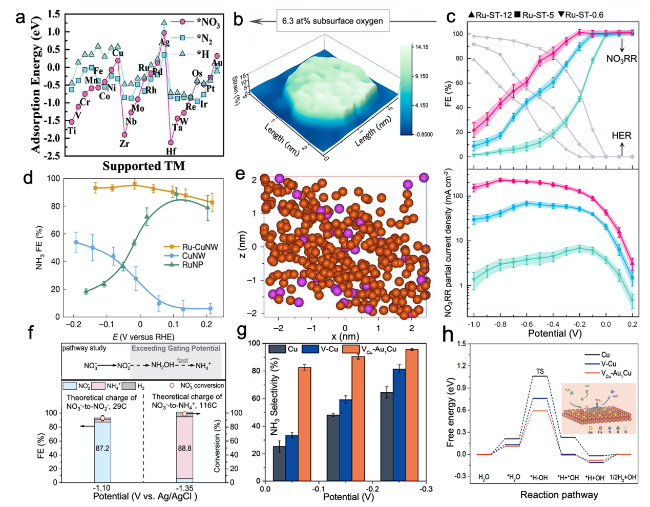
3.1 Noble metal-based electrocatalysts
Fig. 4 a Comparison of NO3-, N2 molecule, and H proton adsorption energies, on TM/g-CN [40]. b Three-dimensional (3D) topographic strain distribution image of Ru-ST-12. c FENH3 and NO3RR partial current densities (JNH3) of the strained nanoclusters for electrocatalytic NO3- to NH3 [54]. d FENH3 of Ru-CuNW, CuNW and RuNP. e Atom probe tomography analysis of Ru-CuNW [23]. f FE and NO3- conversion on OD-Ag [55]. g Selectivity of NH3 on Cu NSs, V-Cu NSs, and VCu-Au1Cu SAAs. h Free energy diagrams of H2O dissociation (inset: schematic illustration of the reaction mechanism for NO3RR on the VCu-Au1Cu SAAs surface) [56] |
4 Non-noble metal-based electrocatalysts
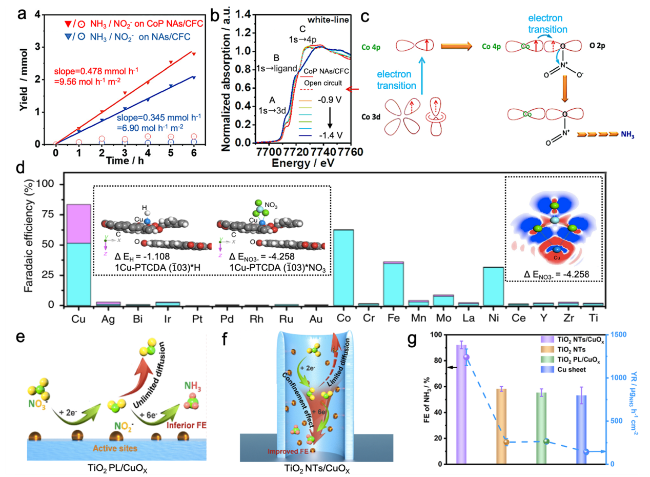
Fig. 5 a Fitting curves of ammonia yield against the electrolysis time. b Operando XANES of CoP NAs/CFC. c NO3RR mechanism on CoP NAs/CFC [66]. d FENH3 (blue) and FENO2- (mauve) of various elements incorporated in PTCDA at -0.4 V vs. RHE. Inset on the left: the adsorption energies of H and NO3- on O-Cu-PTCDA ($\stackrel{-}{1}$03). Inset on the right: the charge density difference of NO3- on 1Cu-PTCDA ($\stackrel{-}{1}$03) [24]. e, f Schematic illustration of NO3RR on (e) TiO2 PL/CuOX and (f) TiO2 NT/CuOX. g FENH3 of TiO2 NT/CuOX, TiO2 NTs, TiO2 PL/CuOX, and Cu sheet at -0.75 V vs. RHE [70] |
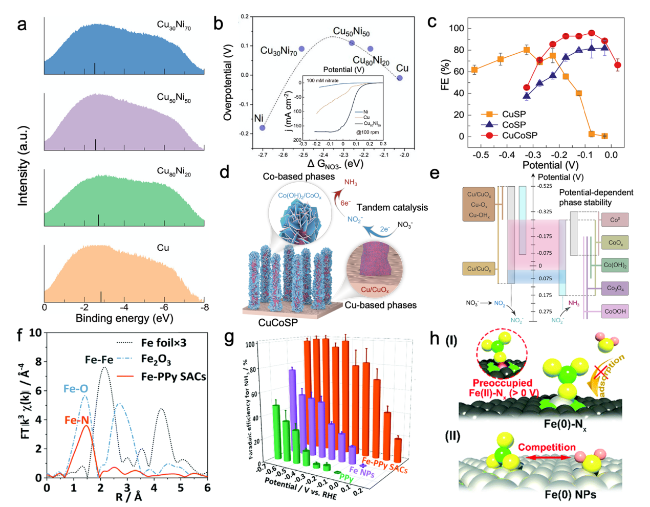
Fig. 6 a UPS spectra of various CuNi alloys and pure Cu catalysts. b The volcano-type relationship between ∆G*NO3 and experimental overpotentials on all CuNi alloys. Inset: current densities of Cu50Ni50 in different concentrations of NO3- [71]. c FENH3 on CuSP, CoSP, and CuCoSP. d Schematic illustration of tandem catalysis of NO3RR on CuCoSP. e The proposed reaction mechanism of tandem catalysis of NO3RR on CuCoSP at low overpotentials [11]. f Fourier transform of extended X-ray absorption fine structure of Fe foil, Fe2O3, and Fe-PPy SACs. g FENH3 on Fe-PPy SACs, Fe NPs, and PPy catalysts. h The proposed mechanism of NO3RR on the single-site center and bulk surface [22] |
5 Metal-free electrocatalysts
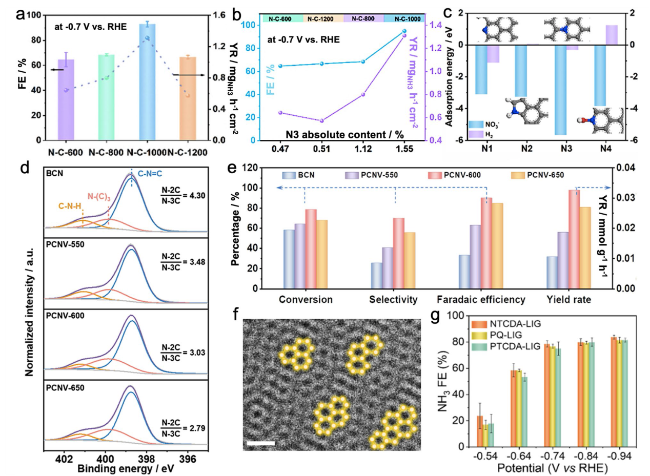
Fig. 7 a FENH3 and YRNH3 of N-C-T at -0.7 V vs. RHE. b The relationship of FENH3, YRNH3, and N3 absolute content. c The adsorption energies of NO3- and H2 [76]. d N 1s XPS spectra of BCN and all PCNV catalysts. e The comparisons of conversion rate, selectivity, FE, and YR of ammonia at -1.6 V vs. RHE over all samples [77]. f Enlargement of High-Resolution TEM images of NTCDA-LIG. g FENH3 of sm-LIGs [78] |
6 Electrocatalysts for the NO2RR
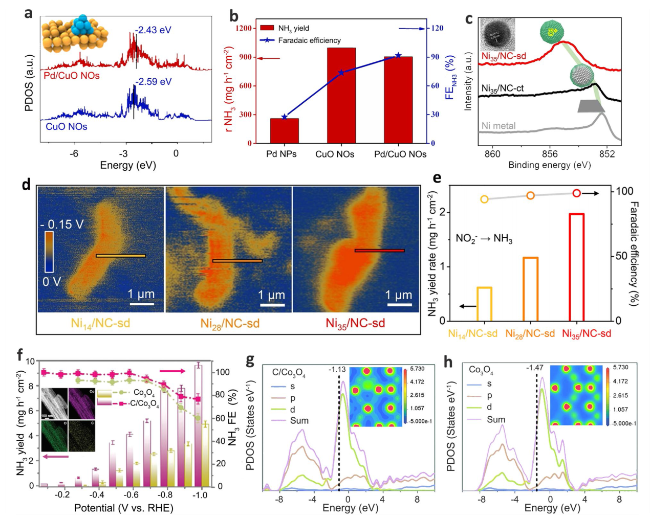
Fig. 8 a The PDOS of Pd/CuO NOs and CuO NOs. b The YR and corresponding FE of Pd/CuO NOs at different potentials [87]. c Ni 2p XPS spectra of Ni35/NC-sd, Ni35/NC-ct and Ni metal plate. d Surface Electric field distribution of Nix/NC-sd samples. e NH3 yield rates and Faradaic efficiency for NO2RR on Nix/NC-sd [82]. f The NO2RR performance of C-Co3O4 at different potentials and the corresponding EDS mapping. g, h PDOS results of C-Co3O and Co3O4 and electron contour maps obtained by KPFM (inset) [41] |
7 Electrocatalysts for the NORR
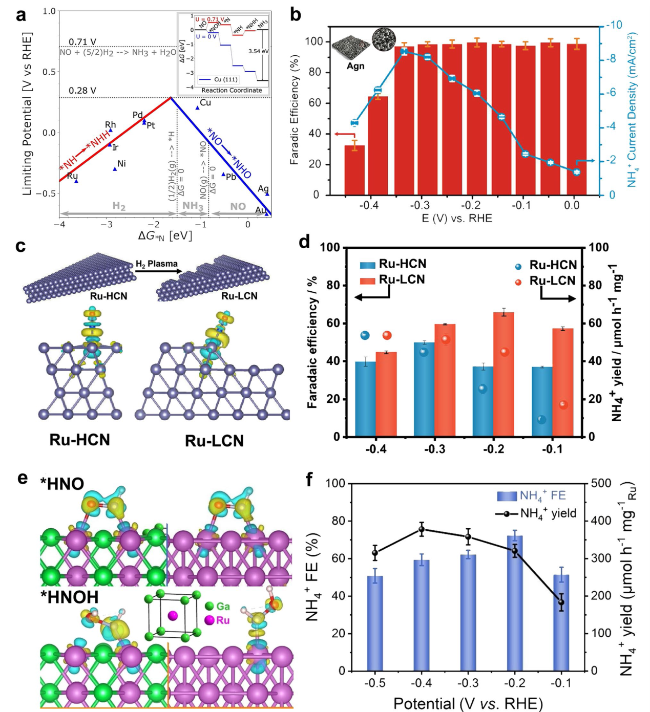
Fig. 9 a The limiting potential volcano for NO reduction to NH3 on different metals [92]. b Effect of applied potentials on FENH4+ and NH4+ partial current density for the Agn electrode in PBS-MC200-NOs electrolytes [98]. c Difference charge density of NO adsorbed by Ru-HCN and Ru-LCN. d FENH4+ and YR NH4+ of 1% NO over Ru-HCN and Ru-LCN at applied potentials [99]. e, f The charge density difference plots of *HNO (e) and *HNOH (f) intermediates adsorbed on bcc RuGa and hcp Ru [100] |
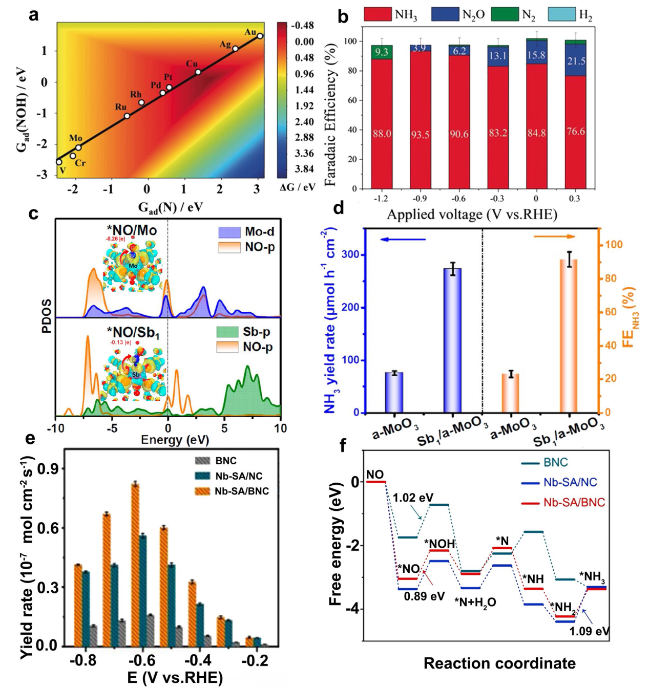
Fig. 10 a A two-dimensional activity map for ammonia production. b FENH3 of NORR on Cu foam at varying potentials [38]. c PDOS profiles and charge density differences (inset) of NO adsorption on a-MoO3 and Sb1/a-MoO3. d FENH3 and YRNH3 of a-MoO3 and Sb1/a-MoO3 at - 0.6 V vs. RHE [101]. e YRNH3 for Nb-SA/BNC at various applied potentials. f Free energy diagrams of adsorbed intermediates for electrochemical NO to NH3. [102] |
8 Conclusion and outlook
Table 1 The performance of different catalysts for NO3RR, NO2RR and NORR |
| Catalyst | Electrolyte | Faradaic efficiency | Ammonia yield rate | Ref. | |
|---|---|---|---|---|---|
| Noble metals for NO3RR | Ru-ST-12 | 1.0 M KOH + 1.0 M KNO3 | > 96% at 0.2 V vs. RHE | 5.56 mol gcat-1 h-1 at -0.8 V vs. RHE | [54] |
| Ru-TiOx/Ti | 1.0 M KOH + 0.5 M NaNO3 | 87.6% at -0.3 V vs. RHE | 2.2 mol g-1 h-1 at -0.3 V vs. RHE | [18] | |
| Ru-CuNW | 1.0 M KOH + 2,000 ppm NO3- | 96.0% at -0.04 V vs. RHE | 76.5 mg h- 1 cm- 2 at -0.135 V vs. RHE | [23] | |
| Rh@Cu-0.6% | 0.1 M Na2SO4 + 0.1 M KNO3 | 93.0% at -0.20 V vs. RHE | 1.27 mmol h- 1 cm- 2 at -0.4 V vs. RHE | [57] | |
| OD-Ag | 0.1 M KCl + 0.1 M NO3- | 88.8% at -1.35 V vs. Ag/AgCl | N.A. | [55] | |
| VCu-Au1Cu SAAs | 1.0 M KOH + 0.1 M KNO3 | 98.7% at -0.2 V vs. RHE | 0.55 mg h- 1 cm- 2 at -0.2 V vs. RHE | [56] | |
| Non-noble metals for NO3RR | CoP NAs/CFC | 1.0 M NaOH + 1.0 M NaNO3 | ∼100% at -0.3 V vs. RHE | 9.56 mol h- 1 m- 2 at -0.3 V vs. RHE | [66] |
| O-Cu-PTCDA | 0.1 M PBS + 500 ppm NO3- | 85.9% at -0.4 V vs. RHE | 4.5 mgNH3 cm- 2 h- 1 at -0.4 V vs. RHE | [24] | |
| CuCoSP | 0.1 M KOH + 0.1 M KNO3 | 93.3% at -0.175 V vs. RHE | 19.89 mgNH3 cm- 2 h- 1 at -0.175 V vs. RHE | [11] | |
| TiO2 NTs/CuOx | 0.5 M Na2SO4 + 100 ppm KNO3-N | 92.23% at -0.75 V vs. RHE | 1.24 mgNH3 cm- 2 h- 1 at -0.75 V vs. RHE | [70] | |
| CuNi alloys | 1.0 M KOH + 0.1 M KNO3 | 99.0 ± 1% at -0.15 V vs. RHE | N.A. | [71] | |
| Fe-PPy SACs | 0.1 M KOH + 0.1 M KNO3 | ~ 100.0% at -0.30 V vs. RHE | 2.75 mgNH3 h- 1 cm- 2 at -0.7 V vs. RHE | [22] | |
| Fe single-atom catalysts | 0.25 M K2SO4 + 0.50 M KNO3 | 75% at -0.66 V vs. RHE | 2.00 mgNH3 h- 1 cm- 2 at -0.85 V vs. RHE | [74] | |
| Metal-free catalysts for NO3RR | N-C-1000 | 0.1 M KOH + 0.1 M KNO3 | 95% at -0.7 V vs. RHE | 1.30 mg h- 1 cm- 2 at -0.7 V vs. RHE | [76] |
| PCNV-600 | 0.5 M Na2SO4 + 100 ppm NO3--N | 89.96% at -1.6 V vs. RHE | 0.03 mmol- 1 g- 1 h- 1 at -1.6 V vs. RHE | [77] | |
| PTCDA-LIG | 1.0 M NaNO3 | 83.7% at -0.94 V vs. RHE | 2.46 mg h- 1 cm- 2 at -0.94 V vs. RHE | [78] | |
| Noble metals for NO2RR | Ag@NiO/CC | 0.1 M NaOH + 0.1 M NO2- | 97.7% at -0.4 V vs. RHE | 5,751 mg h- 1 cm- 2 at -0.4 V vs. RHE | [86] |
| Pd/CuO NOs | 0.1 M 2SO4 + 0.01 M KNO2 | 91.8% at -0.5 V vs. RHE | 0.91 mg h-1$ \mathrm{cm}_{\mathrm{cat}}^{-1} $ at -0.5 V vs. RHE | [103] | |
| Non-noble metals for NO2RR | C/Co3O4 | 0.5 M K2SO4 + 50 mM KNO2 | ~ 100% at -0.6 V vs. RHE | 4.100 mg h- 1 cm- 2 at -0.6 V vs. RHE | [41] |
| Ni35/NC-sd | 0.5 M Na2SO4 + 0.3 M NaNO2 | 99% at -0.5 V vs. RHE | 25.10 mg h- 1 cm- 2 at -0.5 V vs. RHE | [82] | |
| Cu NWs | 0.02 M NaNO2 + 0.1 M KHCO3 | ~ 100% at -1.3 V vs. RHE | 0.60 mg h- 1$ \mathrm{cm}_{\mathrm{cat}}^{-1} $ at -1.3 V vs. RHE | [104] | |
| Cu@TiO2/TP | 0.1 M Na2SO4 + 0.1 M NO2- | 95.3% at -0.6 V vs. RHE | 760.5 µmol h- 1 cm- 2 at -0.6 V vs. RHE | [105] | |
| Noble metals for NORR | Ag | 0.1 M PBS | ~100% | 0.28 mol m- 2 h- 1 | [98] |
| Ru-LCN | 0.5 M Na2SO4 | 65.96% at -0.2 V vs. RHE | 45 µmol h- 1 $ \mathrm{cm}_{\mathrm{cat}}^{-1} $ at -0.2 V vs. RHE | [99] | |
| bcc RuGa | 0.1 M K2SO4 | 72.3% at -0.2 V vs. RHE | 320.6 µmol h- 1$ \mathrm{mg}_{\mathrm{Ru}}^{-1} $ at -0.2 V vs. RHE | [100] | |
| Non-noble metals for NORR | Cu foam | 0.25 M Li2SO4 | 93.5% at -0.9 V vs. RHE | 517.1 mmol h- 1 cm- 2 at -0.9 V vs. RHE | [38] |
| Sb1/a-MoO3 | 0.5 M Na2SO4 | 91.7% at - 0.6 V vs. RHE | 273.5 µmol h- 1 cm- 2 at - 0.6 V vs. RHE | [101] | |
| Nb-SA/BNC | 0.1 M HCl | 95.3% at -0.6 V vs. RHE | 8.2 × 10- 8 mol cm- 2 s- 1 at -0.6 V vs. RHE | [106] |