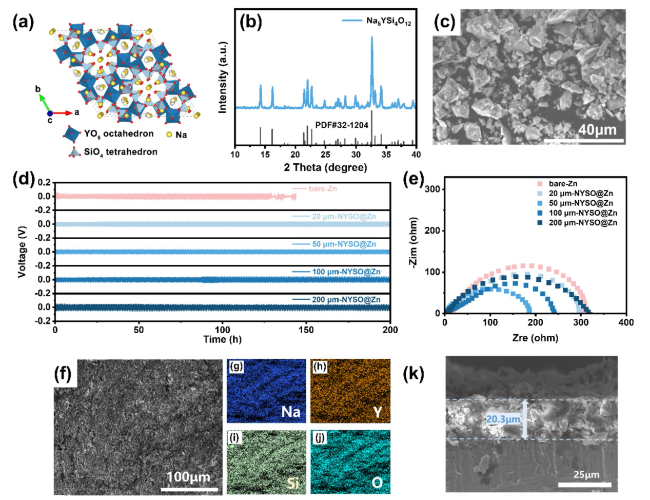
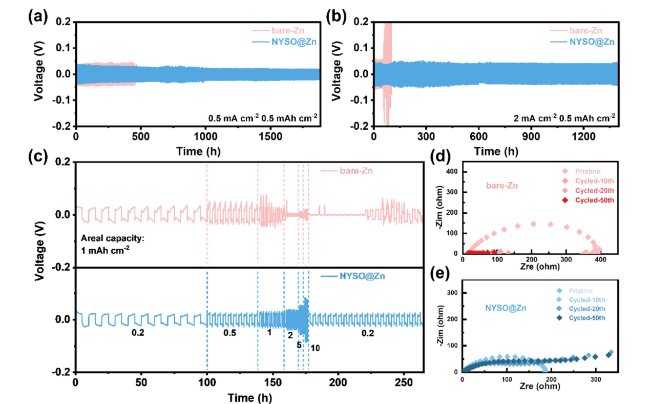
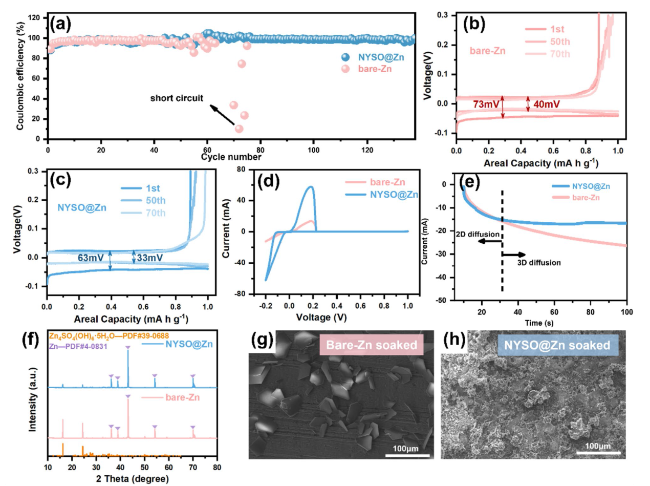
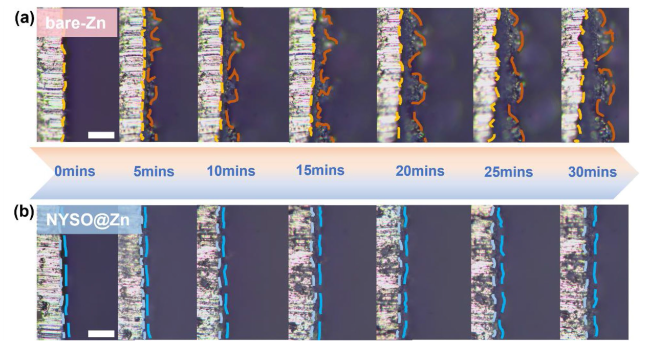
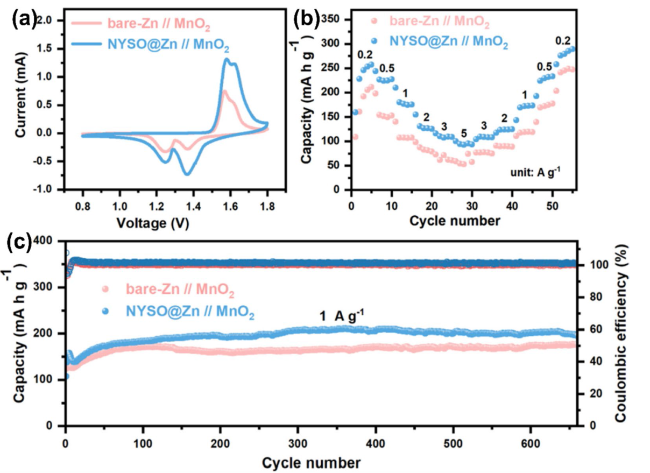
To evaluate the anode stability, the long-term electrochemical performance of the bare Zn and NYSO@Zn symmetric cells were tested at different current densities of 0.5, 1, 2 and 5 mA cm
−2 for 0.5 mAh cm
−2 respectively. Cycled at 1.0 mA cm
−2 with 0.5 mA h cm
−2, the short-circuit induced premature failure occurs for the symmetric cell with bare Zn after 500 h. By contrast, the NYSO@Zn electrode shows ultralow voltage hysteresis (≈30 mV) with negligible voltage oscillation and ultralong lifespan of 1896 h (
Fig. 2a). Under an elevated current density of 1 mA cm
−2, the NYSO@Zn symmetric cell shows prolonged cycling life of 1270 h, which is 9 times that of the bare Zn anode (Figure S2). Besides, comparing the initial surface, the bare Zn suffers deep corrosion and chaos dendrite formation after 100 cycles, whereas the NYSO@Zn maintains even surface morphology, much attributing to the NYSO layer’s ability to restrain the attack of H
2O and realize even Zn
2+ transport flux (Figure S3). The bare Zn symmetric cells experience more rapid failure and show short circuits at cycling times less than 100 and 25 h in 2 and 5 mA cm
−2, which might be due to the rampant Zn dendrites growth at high current densities. However, the NYSO@Zn anodes display smaller overpotentials and stable cycle life up to 1418 and 385 h at 2 and 5 mA cm
−2 as shown in Figs. 2b and S4. In addition, the enlarged voltage-time curves of NYSO@Zn symmetric cells deliver a smaller polarization than those of bare Zn at 0.5 and 2 mA cm
−2 (Figure S5). These results indicate that Na
5YSi
4O
12 coating can achieve uniform Zn
2+ deposition to avoid dendrite formation and extend battery life. In addition, to explore the electrochemical performance of symmetric cells at high current densities, the rate performance was carried out from 0.2 to 10 mA cm
−2 with 1 mAh cm
−2 (
Fig. 2c). As expected, the NYSO@Zn anode presents a favorable rate performance with smaller voltage hysteresis at a high current density of 10 mA cm
−2, whereas the bare-Zn anode shows severe short-circuit at 1 mA cm
−2. The superior cycling life and rate capability can be ascribed to the relatively high Zn
2+ ionic conductivity of the Na
5YSi
4O
12 coating with a controllable interface (Figure S6). As calculated from the Nyquist plot (
Fig. 2e), the charge transfer resistance of the NYSO@Zn electrode in the initial cycle was obviously lower than that of the Zn foil, indicating an efficient charge transfer kinetics. Upon cycling, the NYSO@Zn electrode exhibits similar impedances after 50 cycles, indicating stable interfacial Zn
+ conduction. However, a drastic decrease in charge resistance is observed in the bare Zn cell, suggesting a fundamentally altered charge-conduction mechanism called soft shorts [
32] (
Fig. 2d). In this case, the Zn dendrites cause the small localized electrical connection between two electrodes, which allows the co-existence of direct electron transfer and interfacial reaction, resulting in rapid cell degradation.