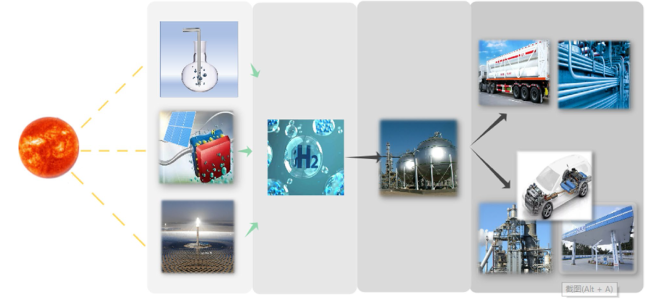
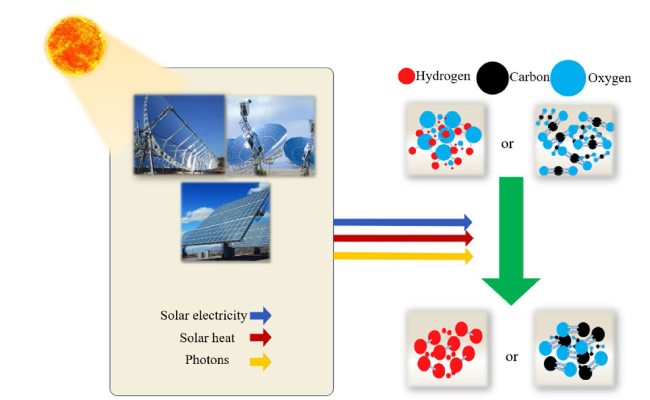
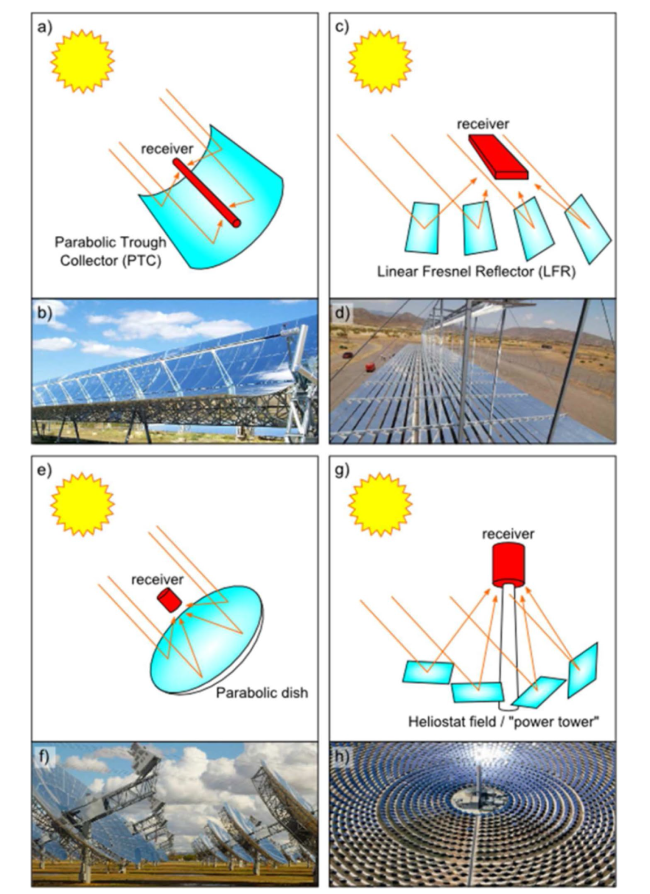
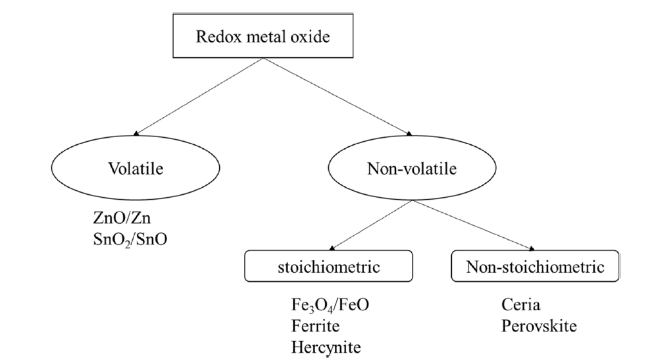
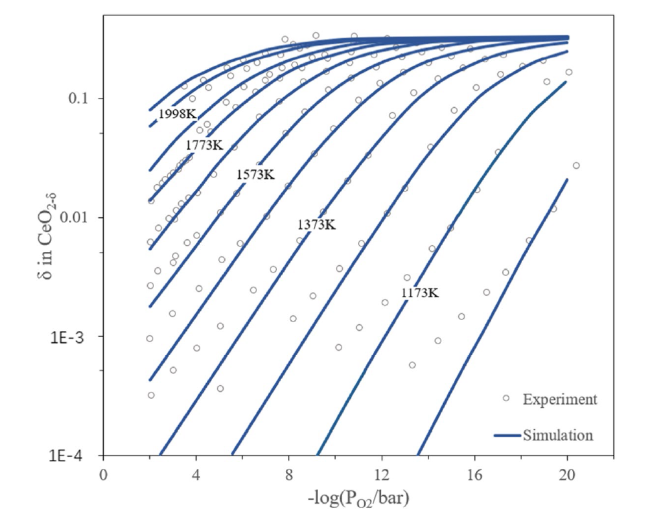
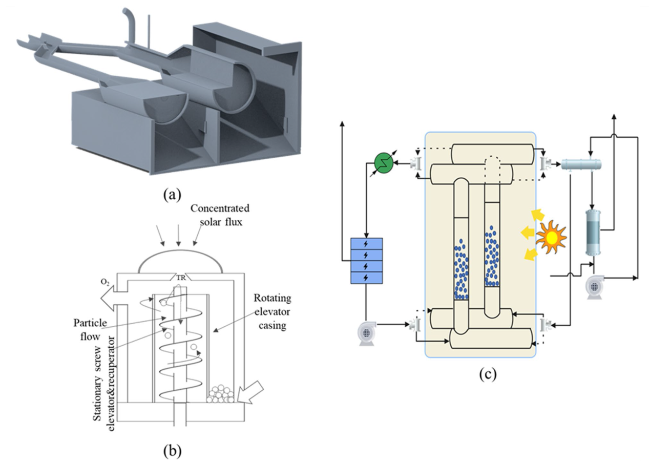
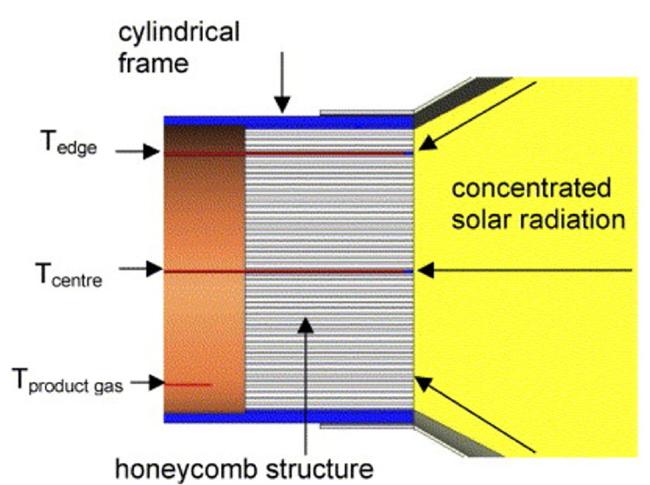
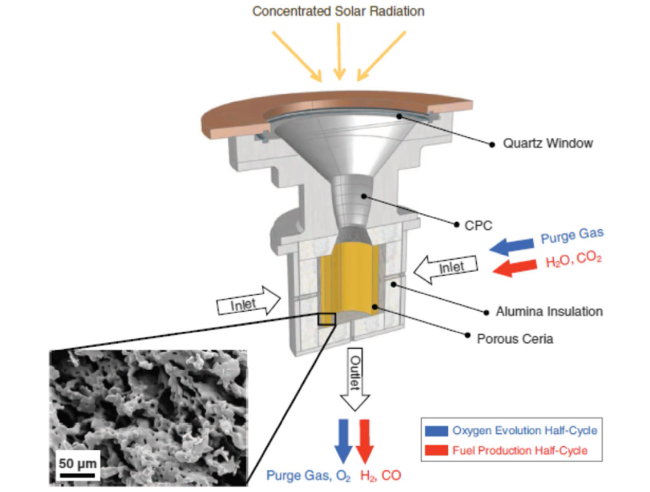
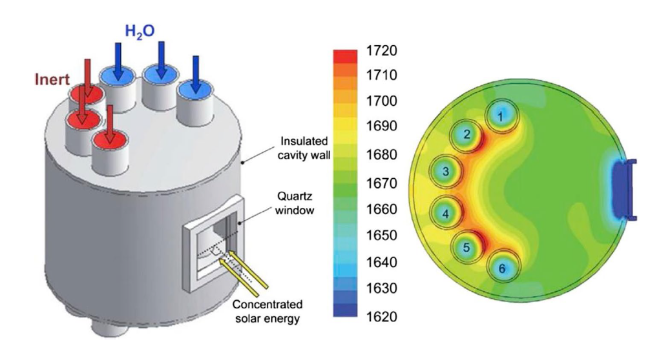
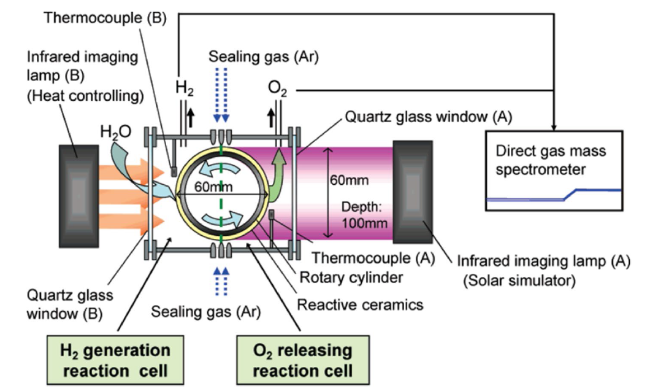
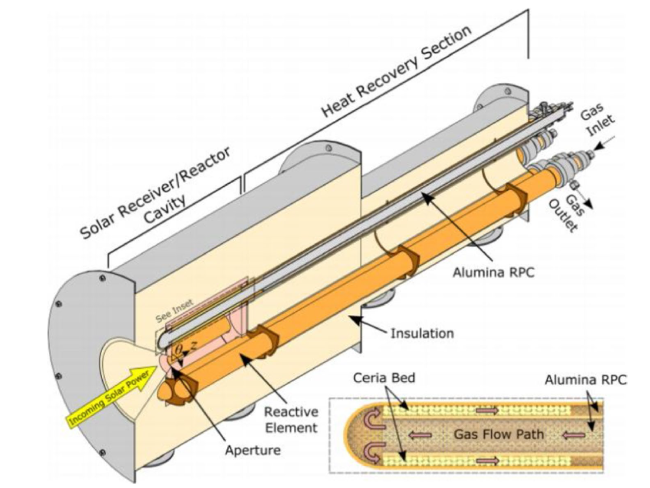
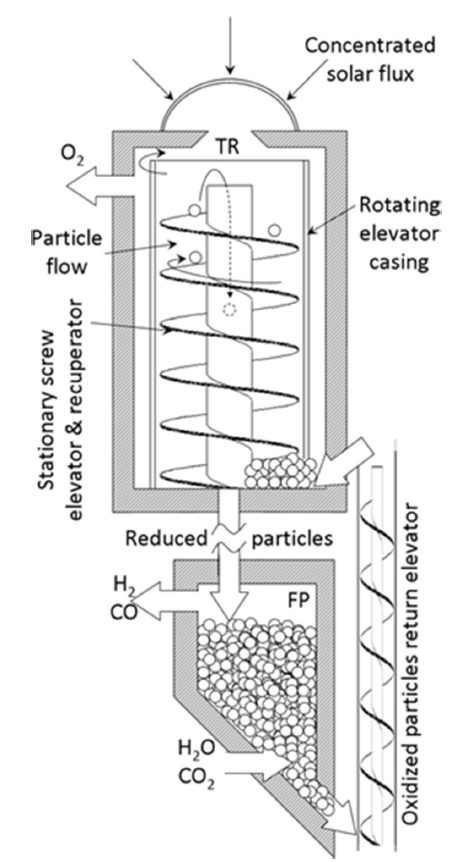
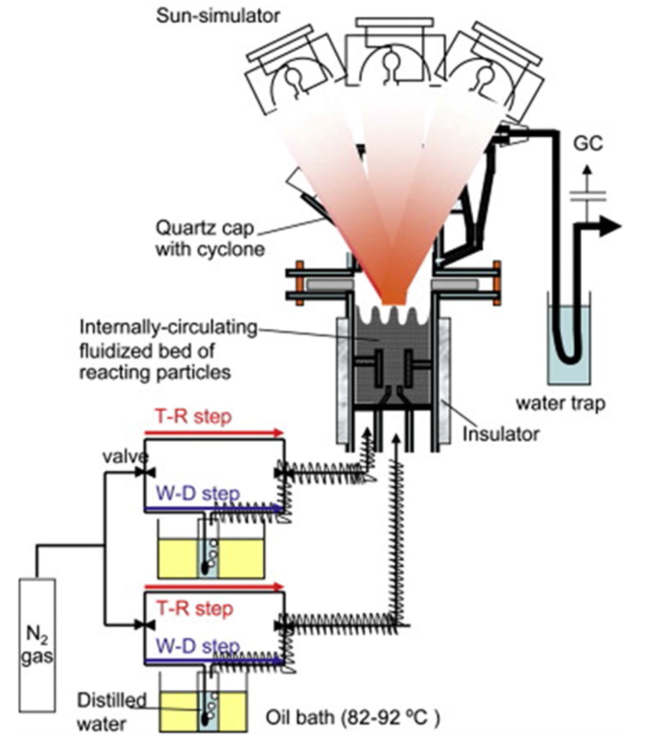
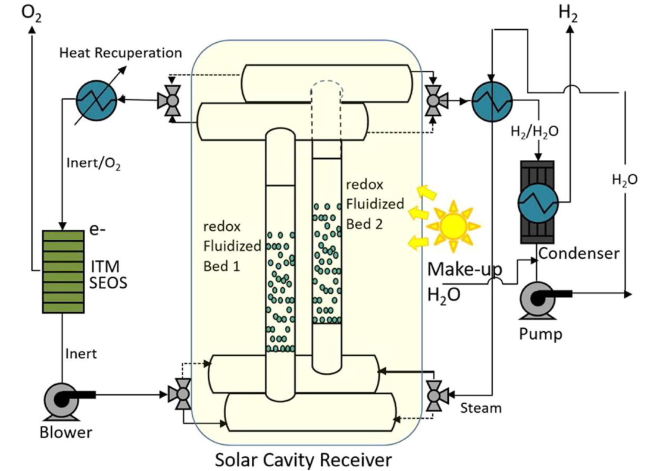
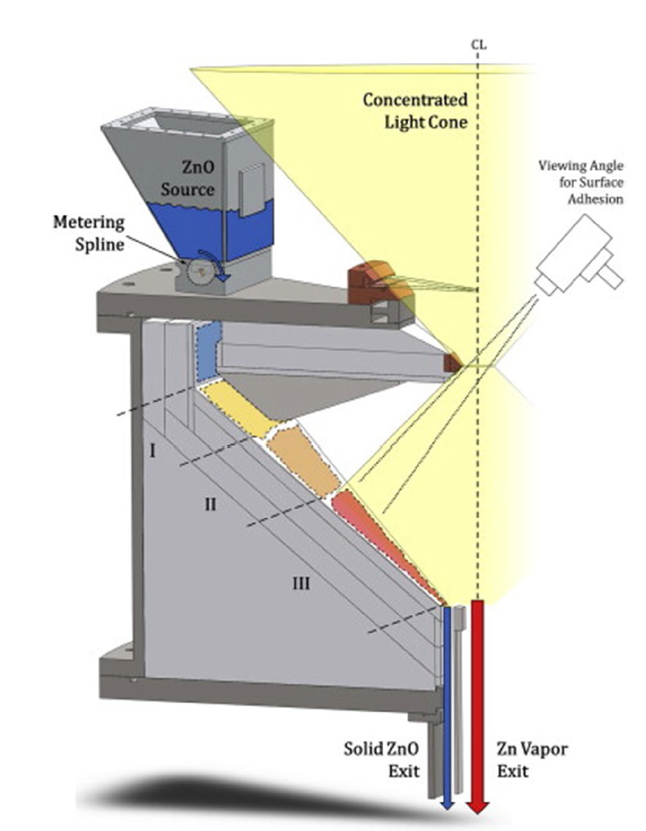
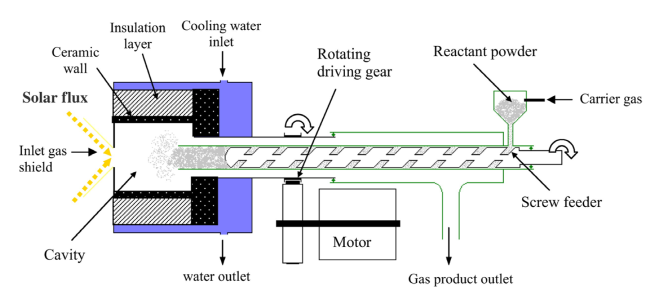
Although the cycle is volatile at 1 bar [
179,
188], it becomes non-volatile at 1700 K under an inert atmosphere, e.g., under nitrogen gas flow. However, it requires another large energy input, e.g., pumping power for the inert sweeping gas. The PROMES group experimentally investigated the performance of the cycle under nitrogen gas flow at 0.1 bar with a solar furnace [
32]. They found that the conversion of the reduction can reach 100%, while the oxidation kinetics is limited by the coagulation due to alternating fusion and solidification of FeO within the cycle. As a result, a granulation is needed in the continuous process, even though it is hard to be implemented. The mixed solid solutions of Fe
3O
4/FeO and M
3O
4/MO (ferrites) can further reduce the reduction temperature [
102]. Lots of ferrites with lower reduction temperatures have been investigated, which are listed in
Table 3. Based on the theoretical thermodynamic analysis [
15], the stabilities of M-ferrite follows the rank of Fe
3O
4 > CoFe
2O
4 ∼ NiFe
2O
4 > ZnFe
2O
4. Moreover, Fresno et al. [
60,
61] experimentally evaluated the activity of commercially available ferrites with different compositions, NiFe
2O
4, Ni
0.5Zn
0.5Fe
2O
4, ZnFe
2O
4, Cu
0.5Zn
0.5Fe
2O
4 and CuFe
2O
4. The net hydrogen production and cyclability after the first reduction-oxidation cycle decreases in the order NiFe
2O
4 > Ni
0.5Zn
0.5Fe
2O
4 > ZnFe
2O
4 > Cu
0.5Zn
0.5Fe
2O
4 > CuFe
2O
4. Among the ferrites in
Table 3, Ni
0.5Mn
0.5Fe
2O
4 has the lowest reduction temperature, while the cyclability is unfeasible. NiFe
2O
4/m-ZrO
2 has the highest hydrogen production rate [
60,
61,
79]. ZnFe
2O
4 is least desirable because of the high temperature for the reduction.