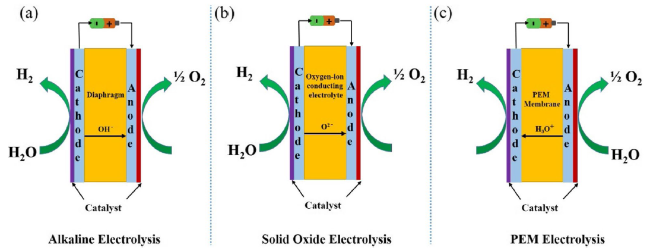
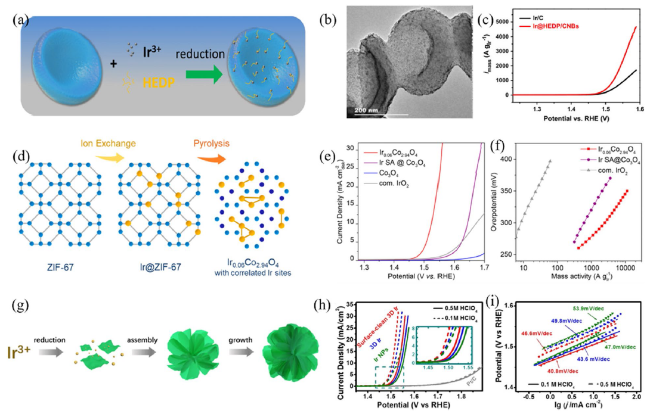
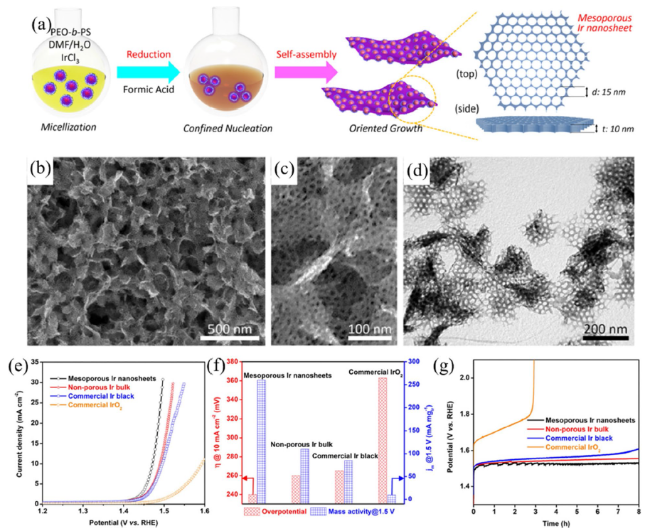
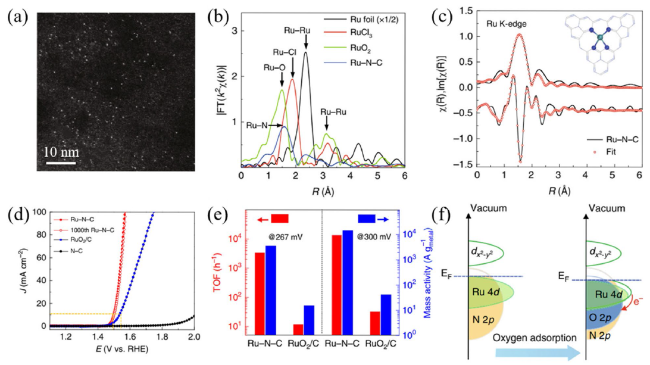
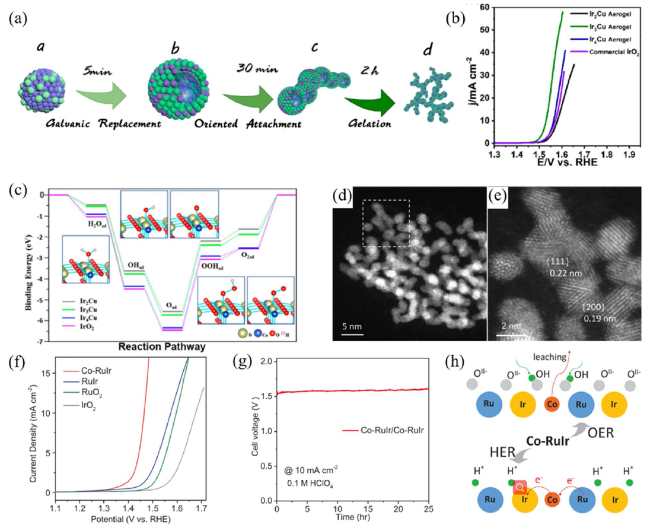
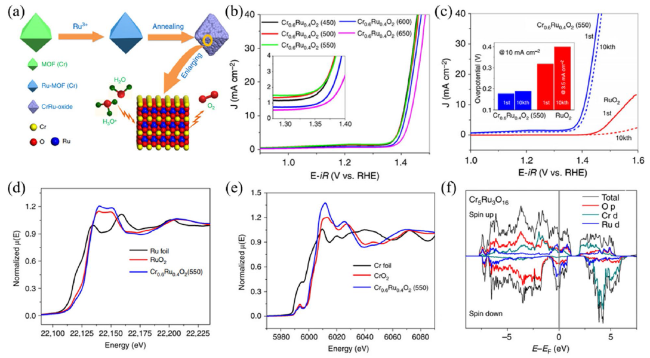
Metal oxide catalysts Compared to metal catalysts, metal oxide catalysts are more active and stable under acidic and oxidizing OER conditions. Hartig-Weiss et al
. [
100] reported an Ir oxide catalyst supported on high surface area and high-conductivity antimony-doped tin oxide, which displayed a very compelling OER activity and reduced the usage of Ir metal, benefitting from the uniform catalyst dispersion and strong metal-support interaction (SMSI). The oxidized Ir quantum dots supported on the surface of graphdiyne (IrO
xQD/GDY) are synthesized and showed incredibly acidic OER performance and robust stability [
74], which only need a small overpotential of 236 mV to reach a current density of 10 mA cm
−2. In this work, GDY could effectively improve the electrical conductivity, facilitate the charge transfer, easy gas releasing and protect the Ir catalysts from corrosion. The early transition metals have a relatively high resistance to corrosion in strong acids [
101], thus, the introduction of early transition metals may have a significant effect on the acidic OER performance. An early transition metal hafnium-modified IrO
x (IrHf
xO
y) was successfully prepared and displayed intriguing OER activity in both alkaline and acid electrolytes [
75]. In order to improve the OER performance of RuO
x, a low-cost rutile-structured chromium-ruthenium oxide catalyst (Cr
0.6Ru
0.4O
2) was prepared through pyrolysis of Cr-based MOF [
76], which unfolded an ultra-low overpotential of 178 mV at 10 mA cm
−2, as shown in
Fig. 9. Combining XANES results and DFT calculations, they found that the presence of electron transfer from Ru to Cr, and the high valence Ru has the plausible ability for the OER. The introduction of Cr could tailor the electron structure of the RuO
2 phase and has a profound effect on optimizing the durability and activity. It is well known that RuO
2 has relatively higher acidic OER activity derived from its appropriate binding ability of oxygen-contained intermediates, but poor stability. Many reports have demonstrated that the unsatisfactory stability is ascribed to the over-oxidation of Ru-based materials and the formation of the soluble Ru
x>4 derivatives [
77,
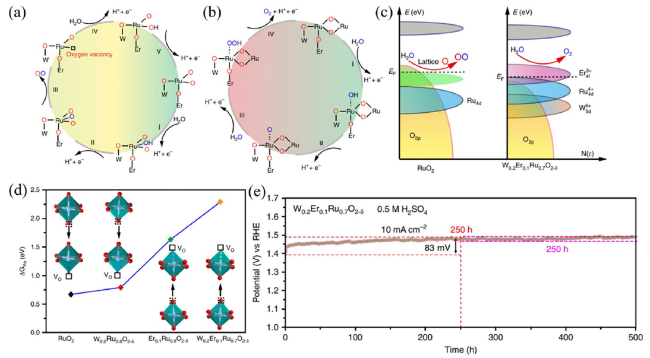
102]. In order to improve the stability, Zhang and co-workers [
77] prepared a bimetallic-doped RuO
x (W
0.2Er
0.1Ru
0.7O
2-δ) catalyst as shown in
Fig. 10. They found that the introduction of W and Er elements could modulate the electron structure of RuO
2 and distinctly increase the formation energy of oxygen vacancy, resulting in an excellent durability of up to 500 h in 0.5 M H
2SO
4, as well as unrivalled OER performance. Because the amorphous/crystalline (
a/c) heterostructure usually unravels unique electronic synergy and exceptionally catalytic performance, Zhang et al
. reported a sodium-decorated
a/c-RuO
2 electrocatalyst [
78]. This
a/c-RuO
2 catalyst displayed a high resistance to acid corrosion and excellent OER activities in all pH environments. Since the relatively higher activity of RuO
2 and much higher stability of IrO
2, preparing IrRu bimetallic oxide or core/shell structure is a promising strategy to form a catalyst with high activity and stability. A highly active and stable RuIrO
x nano-netcage catalyst was reported by Li and co-workers [
79] through a dispersing-etching-holing method using MOF as the template. This open nano-netcage structure with abundant mesoporous could simultaneously improve the atom utilization of Ru/Ir and increase the ECSA. With the unique morphology and component, this RuIrO
x catalyst displayed incredibly startling activity with a low overpotential of 233 mV for reaching 10 mA cm
−2.