1 Introduction
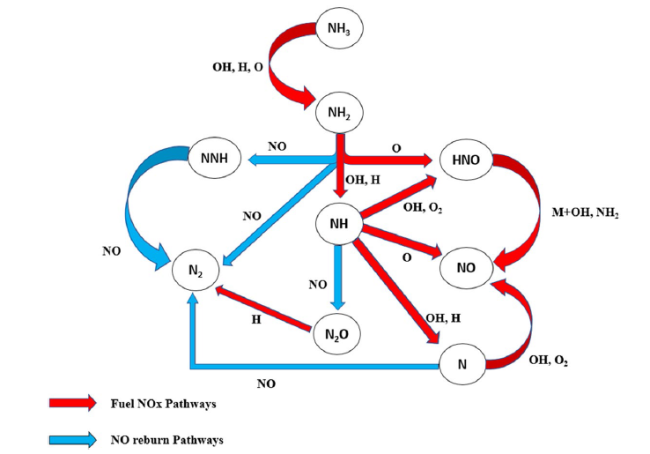
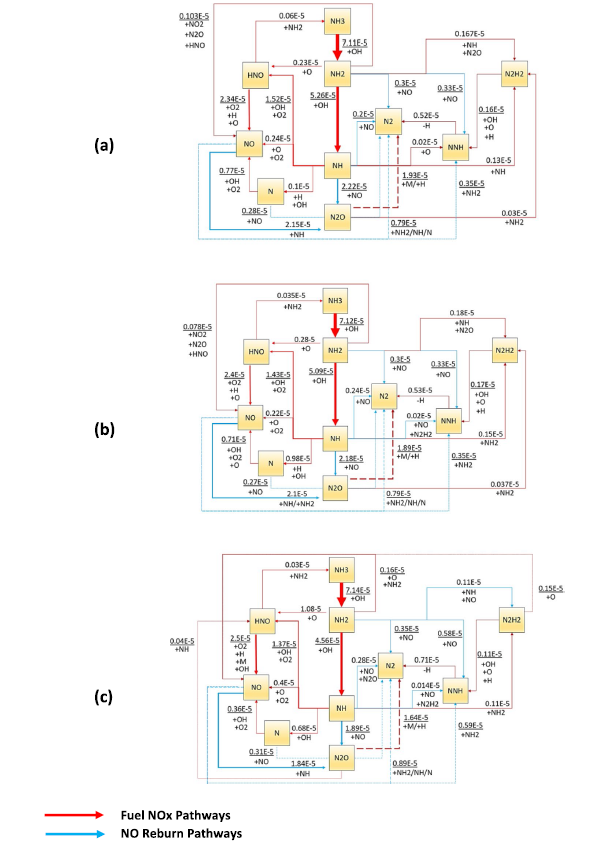
Fig. 1 Reaction pathway diagram illustrating the significant formation/Reburn steps of NO |
2 Methodology
2.1 Experimental work
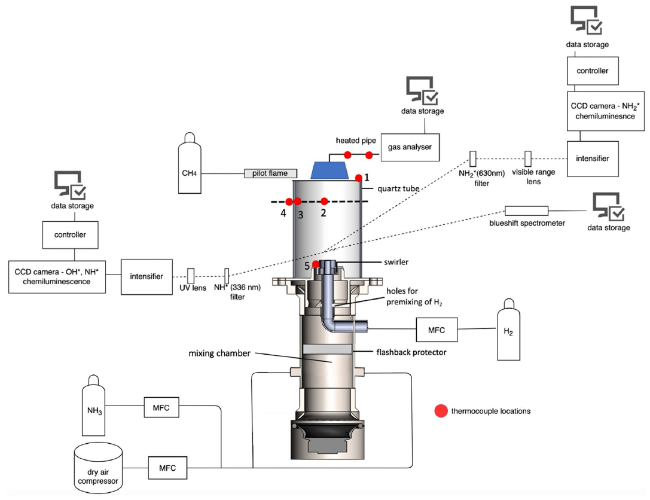
Fig. 2 Tangential burner unit with measuring devices and control systems |
Table 1 Experimental measurements |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Blends NH3/H2 (vol %) | 85/15 | Inlet Temperature | 288 K |
| Thermal Power | 10 kW, 15 kW and 20 kW | Inlet Pressure | 0.11 MPa |
| Reynolds No | 20,000, 30,000 and 40,000 | Outlet Pressure | 0.10 MPa |
| Equivalence Ratio (Φ) | 0.65 | Swirl | 1.05 |
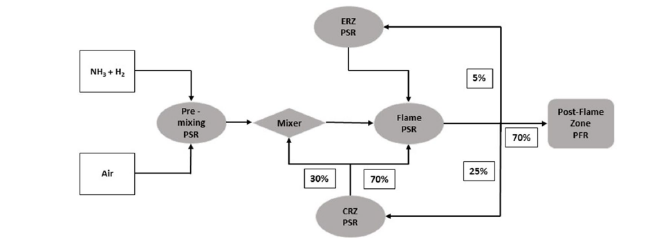
Fig. 3 Chemical reactor network (CRN). CRZ: Central Recirculation Zone; ERZ: External Recirculation Zone |
2.2 Chemical kinetic modeling
Table 2 Grid properties adopted in the current work |
| Number of grid points | 2000 |
|---|---|
| Adaptive grid control of solution gradient | 0.02 |
| Adaptive grid control of solution curvature | 0.02 |
| Starting axial position | 0 cm |
2.3 The investigated kinetic models
Table 3 Chemical kinetic mechanisms used in the present work |
| No | Kinetic model | No. of Reaction | No. of species | Fuel mixture | Optimization parameter | Parameters effect |
|---|---|---|---|---|---|---|
| 1 | A.Bertolino et al. [39] | 264 | 38 | NH3 | LBVs Ignition delay time speciation measurements | Optimization of nitrogen chemistry based on pressure-dependent reactions |
| 2 | B. Mei et al. [40] | 264 | 38 | NH3/NO/N2 | LBVs Markstein length NOx formation | Equivalence ratios |
| 3 | X. Han et al. [41] | 298 | 36 | NH3 NH3/N2O | LBVs speciation measurements | Equivalence ratios N2O mixing ratios |
| 4 | X. Zhang et al. [42] | 263 | 38 | NH3 NH3/H2 | NOx formation | Lean and rich conditions Hydrogen-enriched in fuel |
| 5 | A. Stagni et al. [43] | 203 | 31 | NH3 | LBVs Ignition delay time speciation measurements | Optimization of ammonia oxidation mechanism in a full range of operating conditions |
| 6 | X. Han et al. (2019) [44] | 130 | 20 | NH3 + syngas | LBVs Ignition delay time NOx measurements | Equivalence ratios |
| 7 | S. de Persis et al. [45] | 647 | 103 | CH4 | LBVs NOx measurements | Equivalence ratios Elevated pressure |
3 Results and discussions
3.1 Effects of Reynolds number
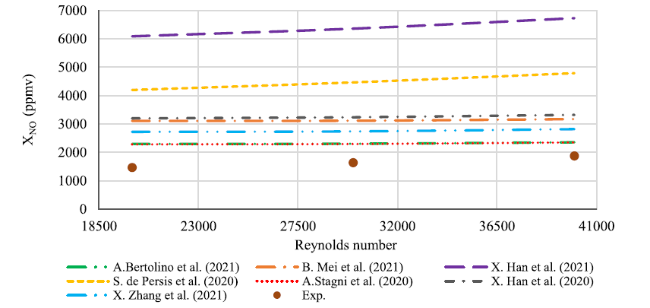
Fig. 4 Comparison between experimental results and numerical concentration profiles of NO when the Reynolds number varies from 20,000 to 40,000 at the exhaust |
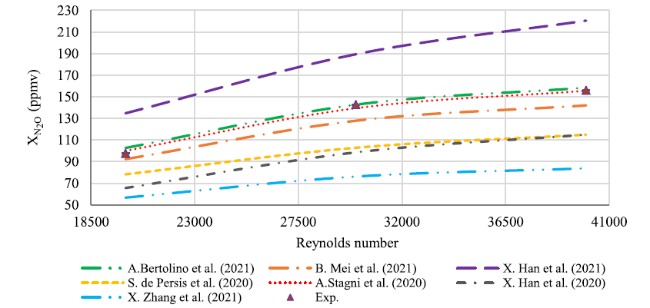
Fig. 5 Comparison between experimental results and numerical concentration profiles of N2O when the Reynolds number varies from 20,000 to 40,000 at the exhaust |
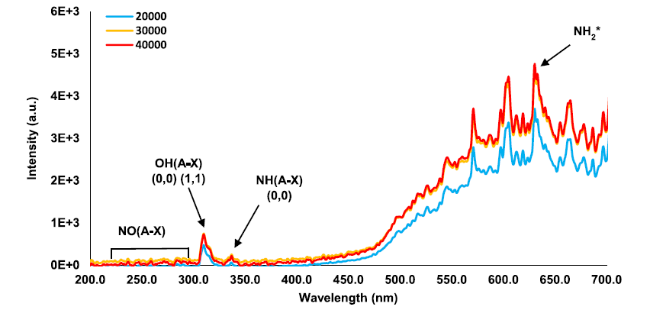
Fig. 6 Chemiluminescence spectrum at Φ = 0.65 and various Reynolds numbers at the flame zone |
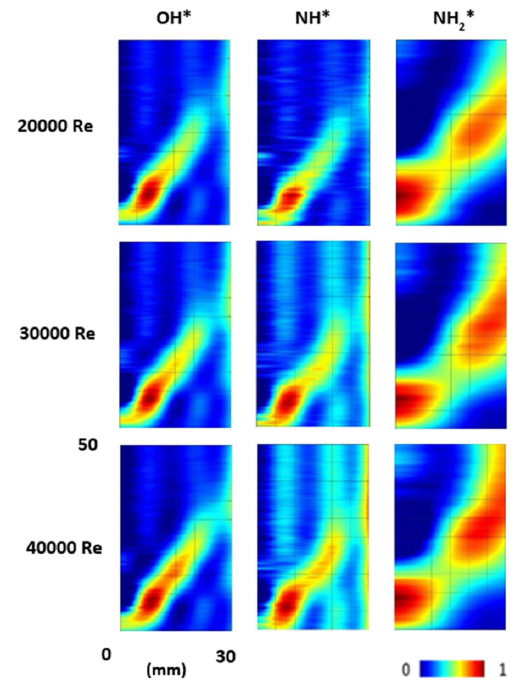
Fig. 7 NH*, NH2*, and OH* chemiluminescence at 85/15 VOL% Of NH3/H2 blend at Φ = 0.65 and various Reynolds numbers at the flame zone. Colormap normalized to image dataset maximum |
Table 4 NO*, OH*, NH* and NH2* values resulted at various Re |
| RADICALS RANGE | Re = 20,000 | Re = 30,000 | Re = 40,000 |
|---|---|---|---|
| NO* (221—261 nm) | 2817 | 4920 | 4941 |
| OH* (302—326 nm) | 10,258 | 13,688 | 14,602 |
| NH* (335—346 nm) | 1552 | 2495 | 2768 |
| NH2* (622—642 nm) | 122,973 | 151,558 | 158,137 |
3.2 Effects of thermal power
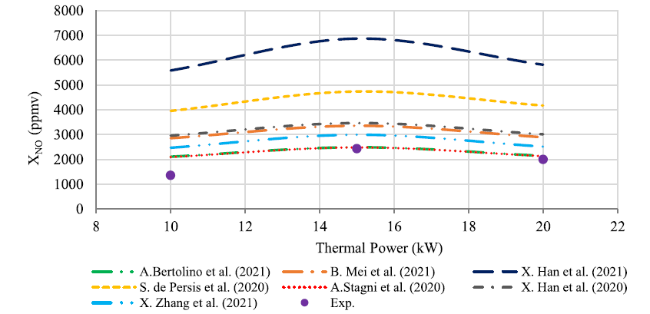
Fig. 8 Comparison between experimental results and numerical concentration profiles of NO when thermal power in rang 10-20kW at the exhaust |
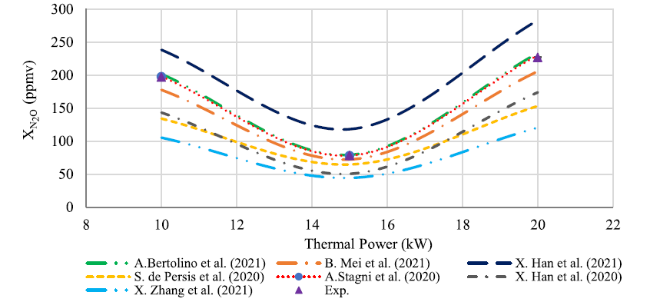
Fig. 9 Comparison between experimental results and numerical concentration profiles of N2O when thermal power in range 10-20kW at the exhaust |
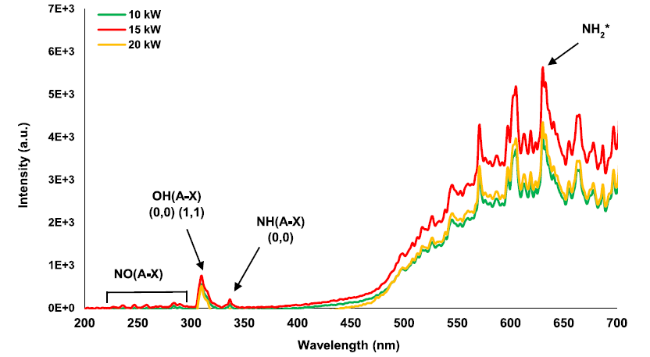
Fig. 10 Chemiluminescence spectrum at Φ = 0.65 and various thermal powers at the flame zone |
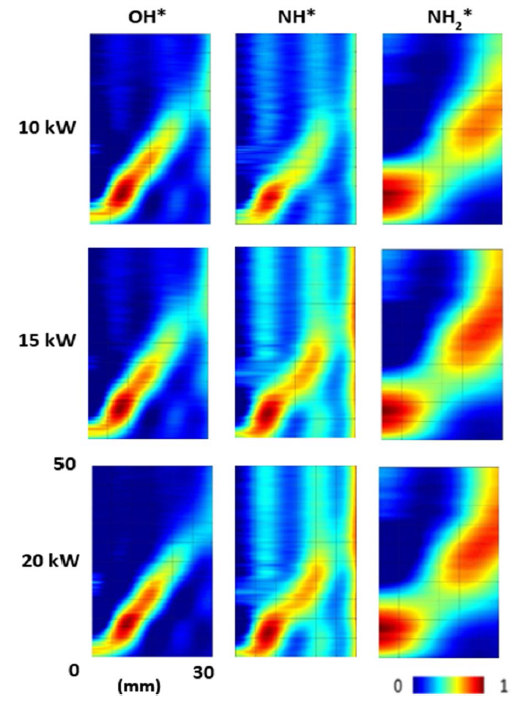
Fig. 11 NH*, NH2*, and OH* chemiluminescence at 85/15 VOL% Of NH3/H2 blend at Φ = 0.65 and various thermal power at the flame zone. Colormap normalized to image dataset maximum |
Table 5 NO*, OH*, NH* and NH2* values resulted at various Re |
| RADICALS RANGE | 10 kW | 15 kW | 20 kW |
|---|---|---|---|
| NO* (221—261 nm) | 3108 | 4720 | 4468 |
| OH* (302—326 nm) | 11,677 | 15,045 | 14,039 |
| NH* (335—346 nm) | 1687 | 2503 | 2405 |
| NH2* (622—642 nm) | 134,210 | 184,198 | 150,000 |
3.3 Sensitivity analyses
3.4 [NO] Sensitivity analysis
3.4.1 [NO] Sensitivity analysis for 20 kW of thermal power
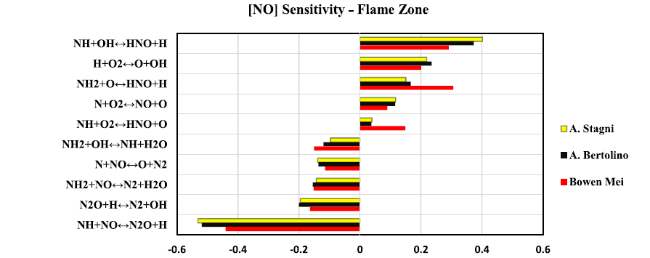
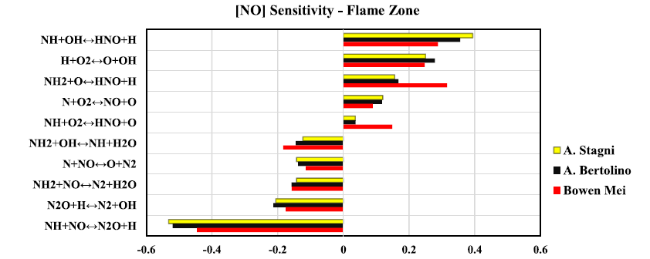
Fig. 12 Sensitivity analysis diagram of NO at the flame zone for 20 kW thermal power |
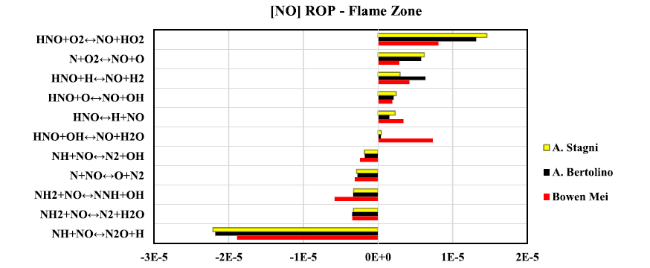
Fig. 13 Rate of production diagram of NO at the flame zone for 20 kW thermal power |
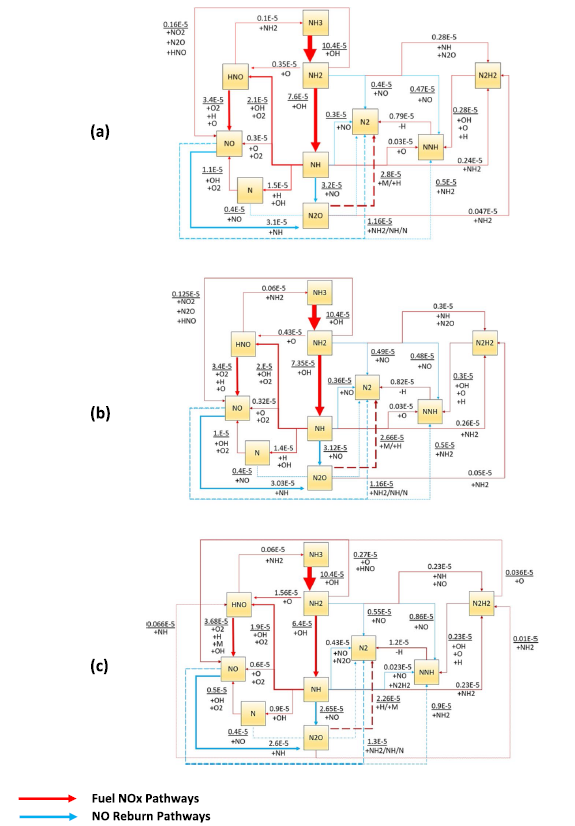
Fig. 14 Chemical reaction pathways show NOX formation/consumption at flame zone when thermal power equal to 20 kW predicted by (a) Stagni, (b) Bertolino, and (c) Mei kinetic models. Lines refer to the reaction path; Numbers stand for the absolute rate of production in ppmv, which has been represented by line thickness for better explanation |
3.4.2 [NO] Sensitivity analysis when Re = 40,000
Fig. 15 Sensitivity analysis diagram of NO at flame zone when Reynolds number equal to 40,000 |
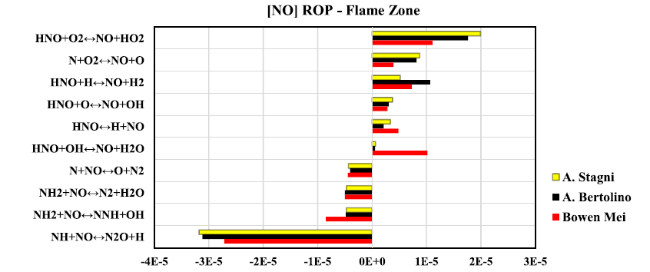
Fig. 16 Rate of production diagram of NO at flame zone when Reynolds number equal to 40,000 |
Fig. 17 Chemical reaction pathways presenting NOx formation/ consumption at flame zone, Reynolds number equal to 40000 predicted by (a) Stagni, (b) Bertolino, and (c) Mei kinetic models.: Lines refer to the reaction path; Numbers stand for the absolute rate of production in ppmv, which has been represented by line thickness for better explanation |
3.5 [N2O] Sensitivity analysis
3.5.1 [N2O] Sensitivity analysis for 20 kW of thermal power
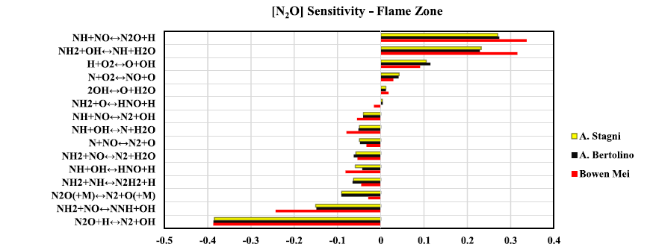
Fig. 18 Sensitivity analysis diagram of N2O at flame zone when thermal power equal to 20 kW |
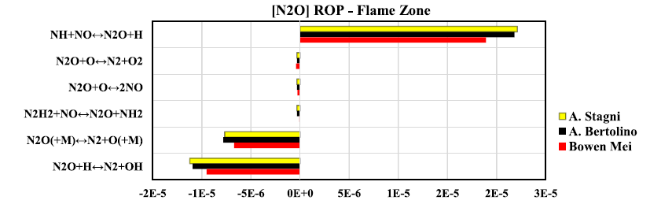
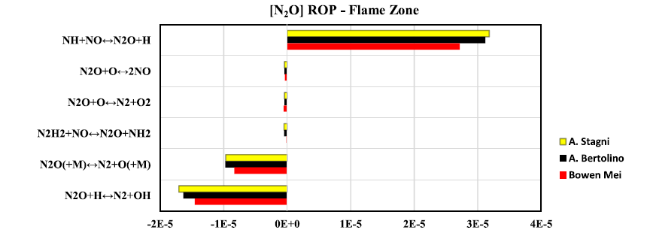
Fig. 19 Rate of production diagram of NO at flame zone when thermal power equal to 20 kW |
3.5.2 [N2O] Sensitivity analysis when Re = 40,000
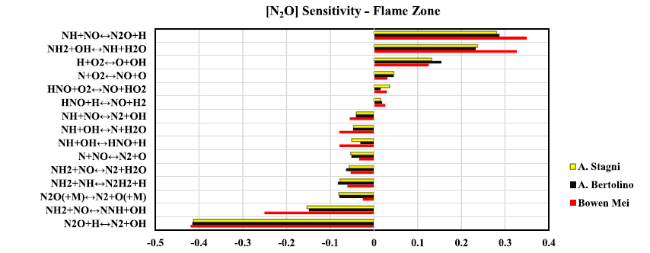
Fig. 20 Sensitivity analysis diagram of N2O at flame zone when Reynolds number equal to 40,000 |
Fig. 21 Rate of production diagram of N2O at flame zone when Reynolds number equal to 40,000 |