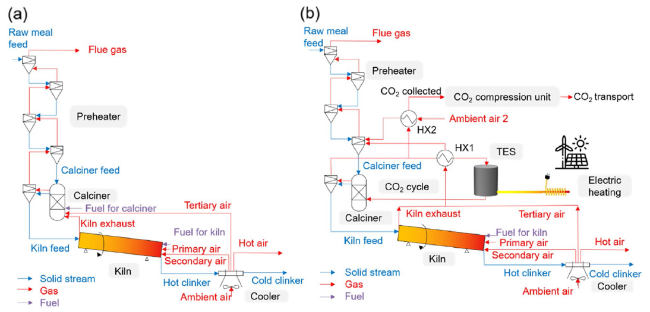
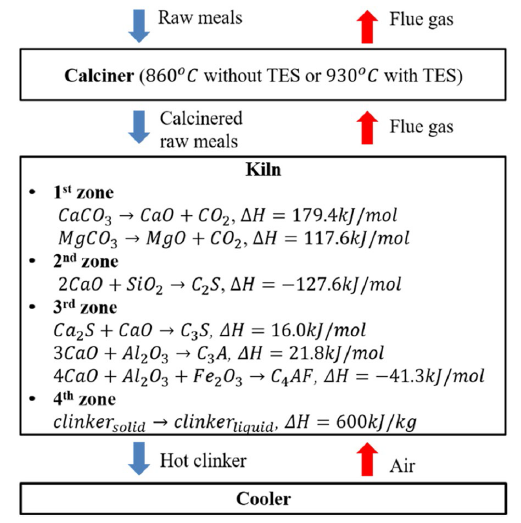
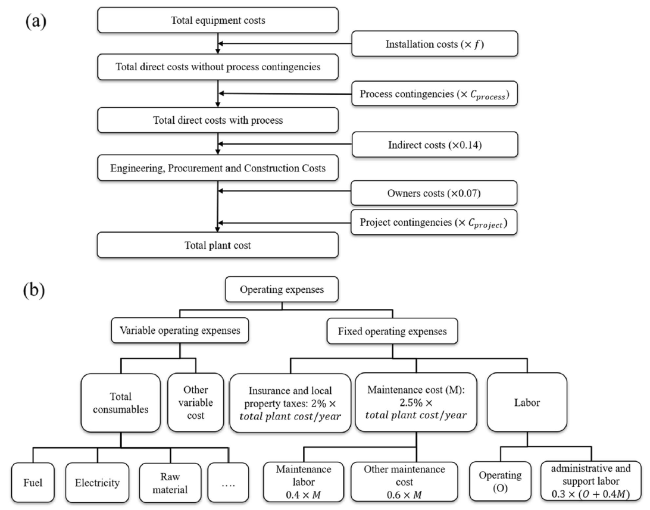
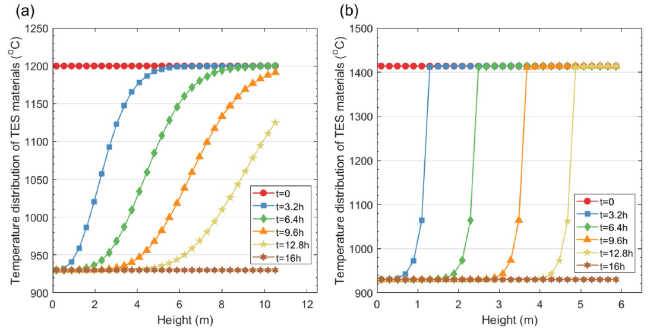
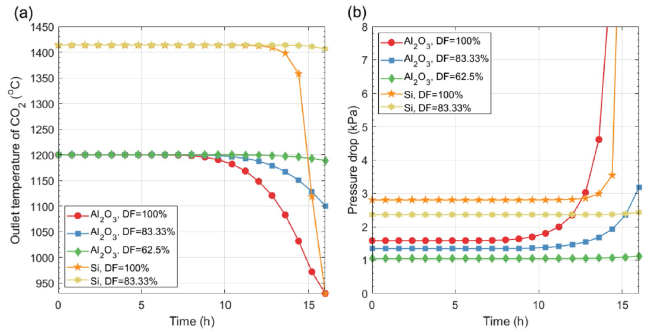
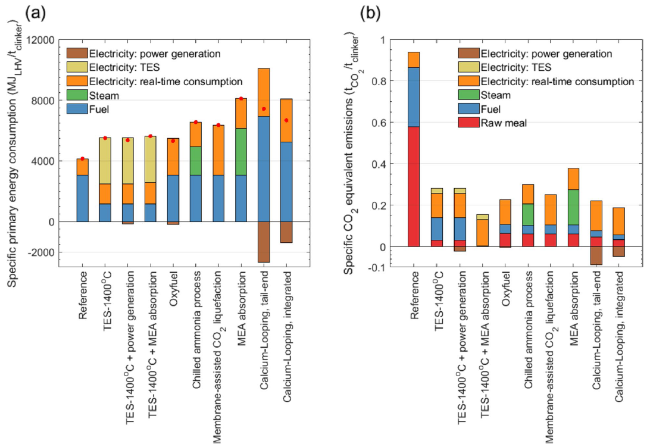
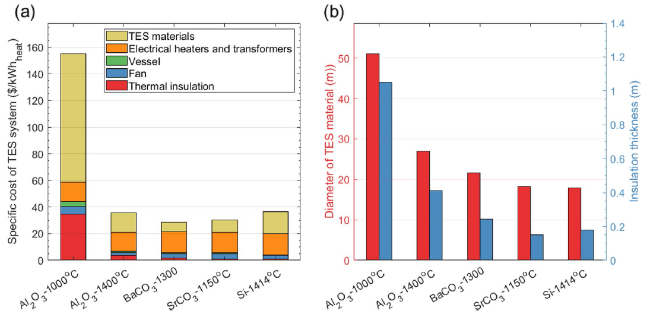
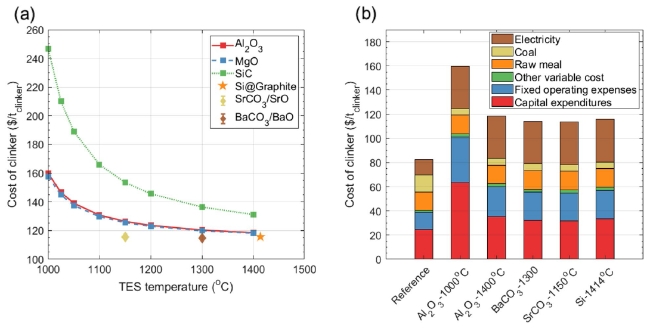
Figure 6 shows the specific energy consumption and CO
2 emissions with different carbon capture technologies. Three TES-based manufacturing processes are presented with different waste heat recovery approaches. The rest of the CO
2 reduction technologies are derived from the EU’s “CO
2 capture from cement production” project [
48]. Since the decomposition ratio of CaCO
3 in the calciner and the outlet temperature of the CO
2 stream are the same, the thermal load in the calciner is the same for different TES temperatures. From the perspective of energy consumption, the TES temperature mainly affects the flow rate of the circulated CO
2 and the power consumption of the circulation fan. When the TES temperature increases from 1000 °C to 1400 °C, the specific energy consumption changes from 5483.5 MJ/t
clinker to 5502.9 MJ/t
clinker. 1400 °C is used for the energy analysis. The waste heat can be used either for electricity generation or can be used as a heat source for other CO
2 capture such as MEA absorption. TES-1400 °C represents the TES-based situation without heat recovery; TES-1400 °C with power generation represents the TES-based situation with waste heat power generation; TES-1400 °C with MEA absorption represents the TES-based situation integrated with MEA absorption as heat recovery. For the TES-1400 °C situation, the specific energy consumption increases from 4070.0 MJ/t
clinker of the reference plant to 5502.9 MJ/t
clinker, with the overall CO
2 emissions decreasing from 0.938 t/t
clinker to 0.282 t/t
clinker. The overall emissions can be summarized as: 0.029 t/t
clinker from CaCO
3 decomposition, 0.110 t/t
clinker from fuel combustion, 0.116 t/t
clinker from the real-time power consumption, and 0.025 t/t
clinker from thermal energy storage. Of these, the former two are direct emissions and can be further reduced through post-combustion carbon capture approaches. TES-1400 °C with MEA absorption provides an effective approach to further reduce CO
2 emissions. In this situation, the waste heat of 21.63 MW can be recovered in steam. Considering the CO
2 flow rate of 15.52 t/h with a mole fraction of 4.57% in the flue gas, the available heat for CO
2 capture amounts to 5.018 GJ/t-CO
2, which increases the CO
2 capture ratio to 98% [
15]. The CO
2 produced in CaCO
3 decomposition and fuel combustion can be significantly captured in the calciner and the MEA absorption processes in TES-based manufacturing. The corresponding modeling results of the MEA absorption with waste heat are provided in
Table 5 in the Appendix. As a comparison, if all the flue gas from the reference plant is sent for carbon capture through MEA absorption, the direct CO
2 emissions are as high as 103.2 t-CO
2/h. Assuming the heat requirement of 3.5 GJ/t-CO
2 in MEA absorption, only 21.6% of the required energy can be provided by the cement plant itself. Therefore, the specific primary energy consumption for CO
2 avoided for the TES-1400 °C and TES-1400 °C with MEA absorption can be calculated as 1.91 GJ/t-CO
2 and 1.98 GJ/t-CO
2, which is less costly of energy compared to other CO
2 capture technologies except for oxyfuel in the figure. The relatively lower energy consumption can be attributed to the fact that no additional energy is required for CO
2 capture and feedstock production, i.e., oxygen required in oxyfuel and calcium-looping. For the situation of TES-1400 °C with power generation, the waste heat recovery power generation can supplement a small percentage of the real-time electricity consumption with a capacity of 36 kWh/t
clinker, and the CO
2 emission reduction is not obvious.