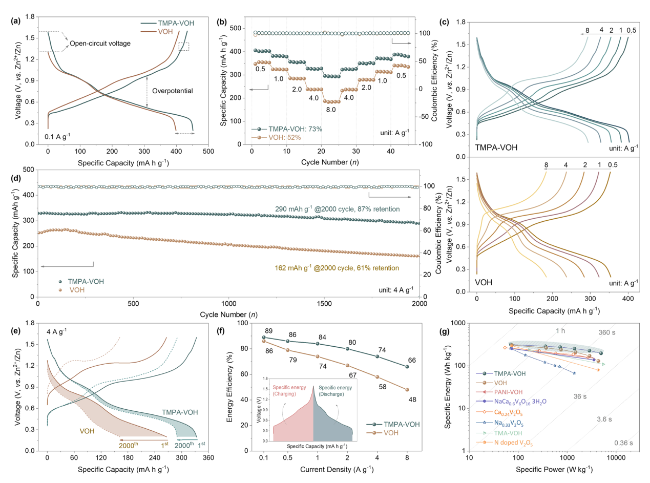
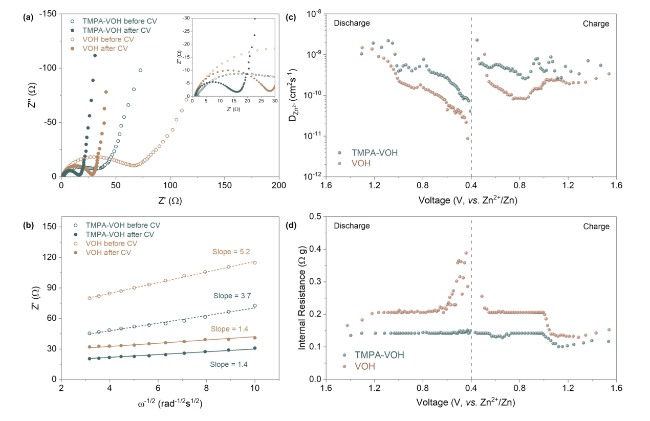
The Galvanostatic intermittent titration technique (GITT) was employed to compare the ion diffusion kinetics of TMPA-VOH and VOH at various charging/discharging stages. The calculated Zn
2+ ion diffusion coefficients (D
Zn2+) during the 3rd GITT cycle at 50 mA g
−1 are presented in
Fig. 6c (calculation details in Supplementary Information). TMPA-VOH exhibits D
Zn2+ values ranging from 9.1 × 10
−11 to 2.4 × 10
−9 cm
2 s
−1 during discharging and 1.9 × 10
−10 to 2.0 × 10
−9 cm
2 s
−1 during charging. In contrast, VOH displays smaller D
Zn2+ values, approximately 8.6 × 10
−12 ~ 1.5 × 10
−9 cm
2 s
−1 during discharging and 8.1 × 10
−11 ~ 1.0 × 10
−9 cm
2 s
−1 during charging. Compared with VOH, the faster Zn
2+ ion diffusion rate of TMPA-VOH throughout the entire intercalation/de-intercalation process indicates that the pre-insertion of TMPA
+ into V-O layers effectively benefits the transport of charge carriers. As demonstrated in
Fig. 6c, both systems exhibit relatively stable diffusivity at the beginning of the discharge process, followed by a rapid decrease after 1.0 V. Initially, the layered structure with large channels allows the “free” insertion of Zn
2+ ions; afterwards, deep intercalation is hindered due to the increasing electrostatic interactions between the inserted Zn
2+. However, this diffusivity decay is more significant in VOH than in TMPA-VOH, especially at lower voltage. This supports the previous statement derived from the CV curves that TMPA-VOH can accommodate more Zn
2+ ions, inserting into the deeper lattice to reduce more V
5+/V
4+ to V
3+. Another important factor, internal resistance, can be estimated from the IR-drop in the GITT results (
Fig. 6d). Internal resistance represents the overall resistance in electrochemical reactions, originating from active materials, current collectors, conductive additives, contact between each element, and electrolyte conductivity. Throughout the charge/discharge process, the internal resistance values for TMPA-VOH are consistently lower than those of VOH (
Fig. 6d), contributing to a reduced overpotential and higher energy efficiency for TMPA-VOH, as confirmed by previous GCD and CV results. During discharging (
Fig. 6d), the internal resistance of VOH displays two steps of increase at ~ 1.2 and 0.6 V, coinciding with the two-step redox reactions upon the Zn
2+ intercalation. The dramatic increase in resistance towards the end of discharge in VOH is a consequence of kinetic limitations, attributed to increased concentration polarization and electrochemical polarization. In contrast, the internal resistance of TMPA-VOH remains relatively stable throughout the entire discharge process, further indicating the reduced electrostatic interactions between the inserted Zn
2+ and the lattice, facilitating the insertion of more Zn
2+ ions into the TMPA-VOH lattice.