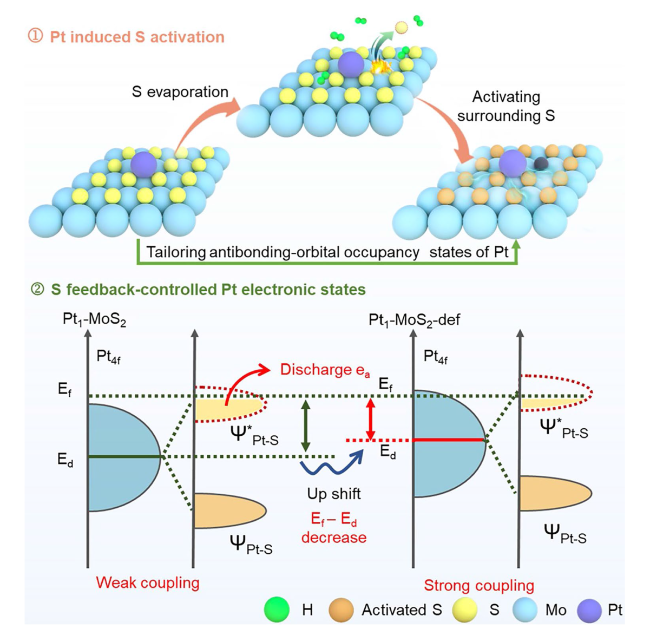
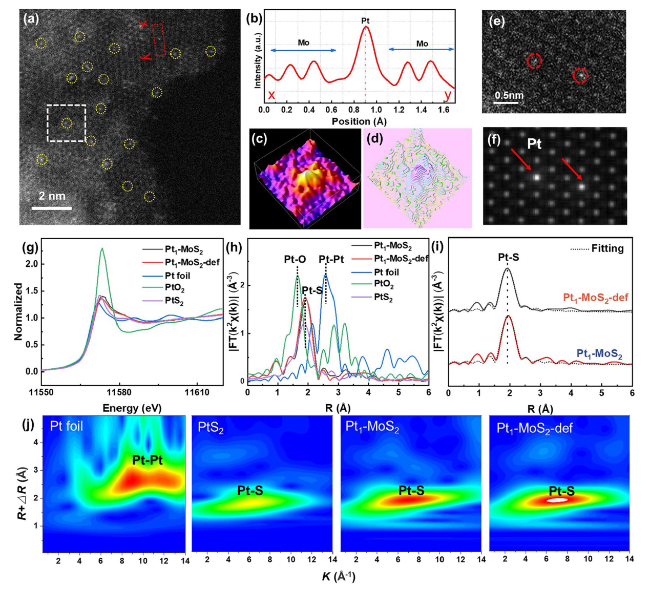
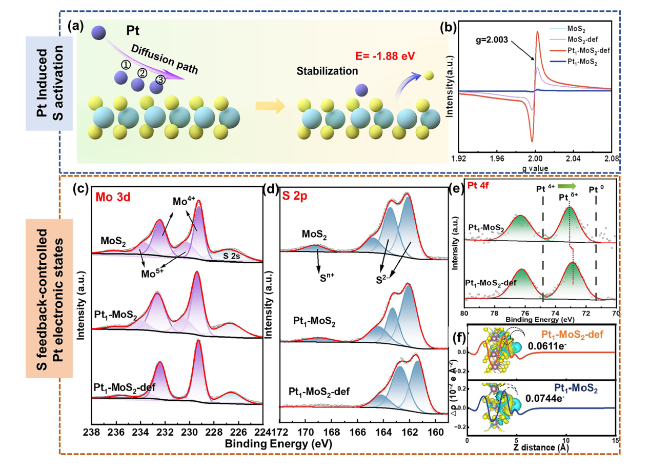
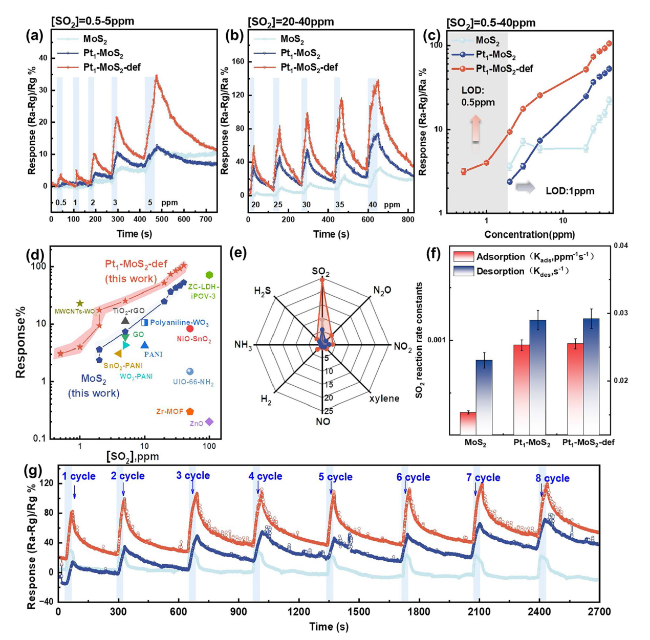
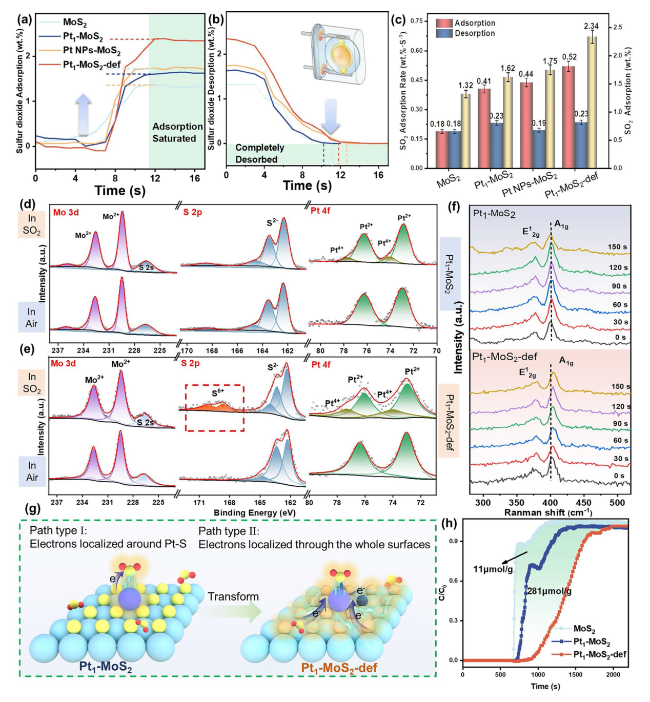
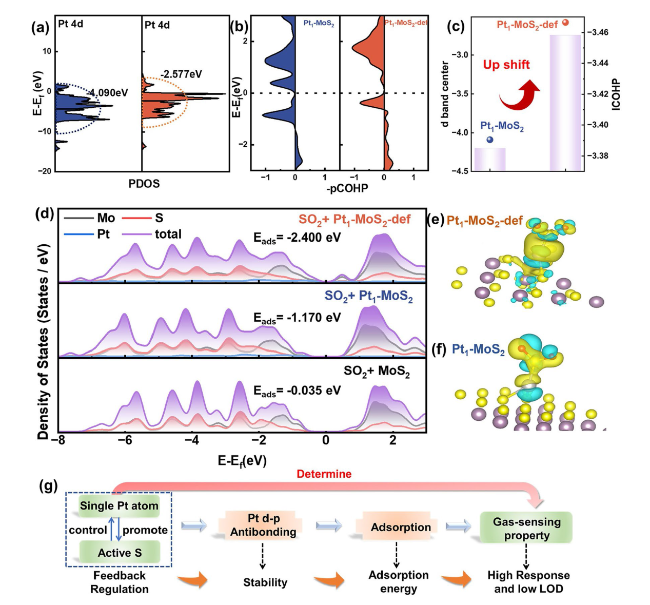
To further clear the design superiority of the Pt
1-MoS
2-def catalysts in the gas-sensing reaction, SO
2 adsorption and desorption tests were implemented (experimental details are shown in Methods and Table S3). As
Fig. 5a-c shows, the Pt
1-MoS
2-def reveals the maximum adsorption capacity (2.34 wt%) and the fastest average adsorption rate (0.52 wt% s
−1) for SO
2 compared with MoS
2 (1.32 wt%, 0.18 wt% s
−1), Pt
1-MoS
2 (1.62 wt%, 0.41 wt% s
−1) and Pt NPs-MoS
2 (1.75 wt%, 0.44 wt% s
−1) at room temperature, indicating the S vacancy-assisted single Pt sites (Pt-Vs) can induce superior adsorption and excitation ability for sulfur dioxide. Ex situ XPS spectra were further employed to investigate the reaction mechanism of different catalysts. As seen in
Fig. 5d, the peaks of Pt
4+ in Pt
1-MoS
2 suddenly appear in the SO
2 atmosphere while no obvious new peaks are found in the Mo and S species, confirming the electron transfer path is from the single Pt site to adsorbed SO
2 molecule. However, a small number of surface-adsorbed SO
2 molecules are not distinguished by the XPS detection. Same results can also be found in MoS
2-def catalysts in Fig. S24. Considering the resistances of MoS
2-based sensors significantly reduce in the oxidizing SO
2 atmosphere (Fig. S25), the sensing type can be identified as p-type semiconductors. Detailly, the SO
2 species can capture electrons from the conduction band of p-type semiconductors and induce the hole generation, resulting in decreased resistances (Fig. S26). For the Pt
1-MoS
2-def catalyst (
Fig. 5e), two new higher-valence S peaks (168.83 and 170.1 eV, S
6+) and Pt
4+ peaks are discovered after SO
2 treatment while there is a slight high binding energy shift in the Mo 3
d spectrum, revealing that both Pt and MoS
2 supports lose electrons during the gas-sensing process. The in situ Raman spectra display the 150 s exposure periods of Pt
1-MoS
2-def, Pt
1-MoS
2 (
Fig. 5f) and MoS
2-def (Fig. S27) after treating with 1000 ppm SO
2. Clearly, the A
1g peak (at 402.25 cm
−1) of the Pt
1-MoS
2-def sample shifts to a higher wavenumber after SO
2 exposure for 60 s, while no peak changes for the Pt
1-MoS
2 and MoS
2-def samples. Therefore, this unequivocally corroborates that the electronic transform process in Pt
1-MoS
2-def involves the adsorbed SO
2 molecules and the whole supports. In conclusion, experimental results demonstrate there are two potential reaction paths dominant to the SO
2 sensing performance for Pt
1-MoS
2-def and Pt
1-MoS
2 sensors. For Pt
1-MoS
2 (
Fig. 5g, type I), the electrons mainly locate around the single Pt site and the electron transfer only occurs within Pt-S (SO
2). In comparison, the Pt
1-MoS
2-def sensors contain synergistically single Pt sites and activated inert S plane, which prompts the whole supporting surface to participate in the SO
2 sensing process (
Fig. 5g, type II). Meanwhile, the rapid electron transfer between Pt
1-MoS
2-def and the oxidizing SO
2 guarantees high response and low LOD. In addition, the in situ SO
2 adsorption breakthrough curves (
Fig. 5h) were implemented to quantitatively measure the absorption of SO
2, in which the adsorbed SO
2 amounts on Pt
1-MoS
2-def is 281 μmol g
−1 more than that on Pt
1-MoS
2, and there is 292 μmol g
−1 more than that on MoS
2-def at 25 °C, according well with the SO
2 adsorption and desorption tests in
Fig. 5a-c.