HIGHLIGHTS
1 Introduction
Fig. 1 Summary of the various challenges of LIBs and ASSLSBs and corresponding strategies to accelerate the commercialization of ASSLSBs |
2 Need for All-Solid-State Lithium-Sulfur Batteries
2.1 Safety Concerns
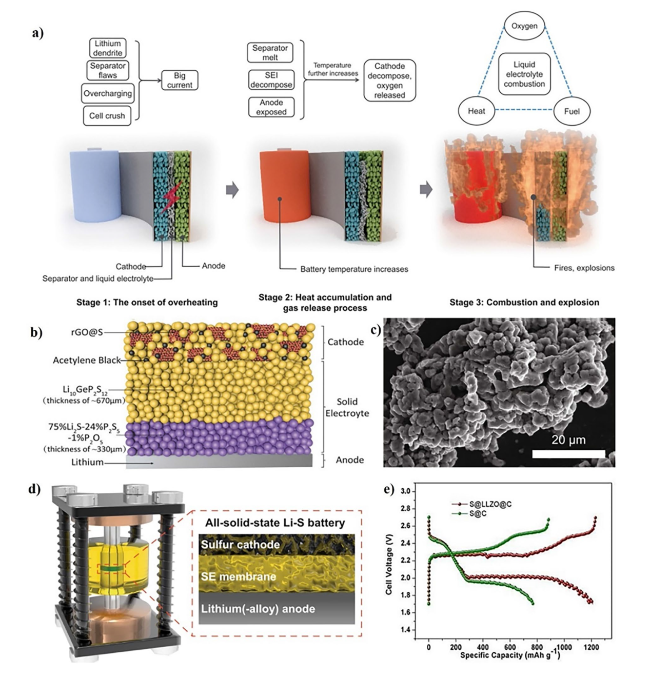
Fig. 2 a Three stages delineate the thermal runaway process of LIBs. Copyright 2018, American Association for the Advancement of Science [34]. b Schematic diagram of an ASSLSB. Copyright 2017, WILEY-VCH [39]. c SEM image and d Schematic illustration of the Swagelok cell and the configuration of Li-S ASSBs. Copyright 2023, The Author(s), Springer Nature Limited [40]. e Typical charge/discharge curves of the S@LLZO@C and S@C cathodes with a current density of 0.1 mA cm.−2 at 50 °C. Copyright 2017, American Association for the Advancement of Science [41] |
2.2 Growing Demand for Higher-Energy-Density Batteries
Table 1 Energy densities of recently reported ASSLSBs |
| Battery design [Cathode ∣Electrolyte ∣Anode] | Capacity [mAh g−1] | Average voltage [V] | Energy density [Wh kg−1] | References |
|---|---|---|---|---|
| MoS6-CNT20@15%Li7P3S11 ∣ Li6PS5Cl ∣ Li | 1034.32 | 1640 | [57] | |
| Li2S ∣ PEO-based electrolytes ∣ Li | 1133 | 416 | [58] | |
| LiCoO2 ∣ 78Li2S·22P2S5 ∣ In | 112 | 3.1 | 10.9 | [59] |
| LiCoO2 ∣ Li3PS4 ∣ In | 150 | 3.1 | 11.4 | [60] |
| F@NMC811∣Li6PS5Cl-Mg16Bi84 ∣ Li | 200 | 4.3 | 310 | [61] |
| LiCoO2∣ Li10GeP2S12∣ Graphite | 104 | 2.2 | 14.6 | [62] |
| S-3DG@SMC ∣ SMC ∣ Li | 1680 | 588.8 | [63] | |
| S/PAN ∣ LCE ∣ Li | 588 | 2.7 | 116 | [64] |
| LiCoO2 ∣ Li10GeP2S12 ∣ In | 112 | 3.1 | 19.2 | [65] |
| LiCoO2 ∣ Li10GeP2S12 ∣ In | 140 | 3.1 | 20.9 | [66] |
| Co3S4 ∣ polydopamine-coated Li6PS5Cl ∣ Li | 485.1 | 284.4 | [67] | |
| LiNi1/3Co1/3Mn1/3O2 ∣ Li6PS5Cl ∣ In | 44 | 3.3 | 9.4 | [68] |
| LiNi1/3Co1/3Mn1/3O2 ∣ Li6PS5Br ∣ In | 109 | 3.3 | 27.0 | [69] |
| LiNi0.8Co0.1Mn0.1O2 ∣ Li3PS4 ∣ In | 124 | 3.1 | 22.5 | [70] |
| LiNi0.8Co0.15Al0.05O2 ∣ 80Li2S·20P2S5 ∣ Graphite | 120 | 3.7 | 40.0 | [71] |
| LiNi0.5Mn0.5O2 ∣ 95(0.6Li2S·0.4SiS2)·5Li4SiO4 ∣ In | 70 | 3.1 | 10.4 | [72] |
| MoS5@10%graphene15%Li7P3S11∣Li6PS5Cl ∣ Li | 570.7 | 470.3 | [73] | |
| FeS2 ∣ Na3PS4/Na3SbS4 ∣ Na3Sn | 346 | 1.68 | 14.4 | [74] |
| NaS2 ∣ Na3PS4 ∣ Na-Sn-C | 869.2 | 1.68 | 8.1 | [75] |
| NaS2 ∣ Na3PS4 ∣ Na-Sn-C | 1050 | 1.68 | 11.8 | [76] |
| S∣Li3.25Ge0.25P0.75S4∣Li-In | 1200 | 1.68 | 18.9 | [77] |
| LiCoO2 ∣ LiPVFM/LiODFB ∣ Li | 133 | 3.7 | 359 | [78] |
2.3 Raw Material Supplies and Sustainability Challenges
Table 2 Prices of selected battery materials. |
| Year | Metal price (GBP) | ||||
|---|---|---|---|---|---|
| Lithium carbonate | Cobalt | Nickel | Copper | Manganese | |
| 2015 | 45 | 80 | 153 | 92 | 103 |
| 2016 | 63 | 59 | 87 | 76 | 56 |
| 2017 | 100 | 100 | 100 | 100 | 100 |
| 2018 | 157 | 216 | 137 | 118 | 123 |
| 2019 | 106 | 92 | 125 | 103 | 112 |
| 2020 | 66 | 94 | 129 | 94 | 89 |
| 2021 | 65 | 111 | 178 | 131 | 95 |
| 2022 | 310 | 191 | 231 | 160 | 103 |
| 2023 | 563 | 131 | 305 | 153 | 92 |
3 Fundamentals of All-Solid-State Lithium-Sulfur Batteries
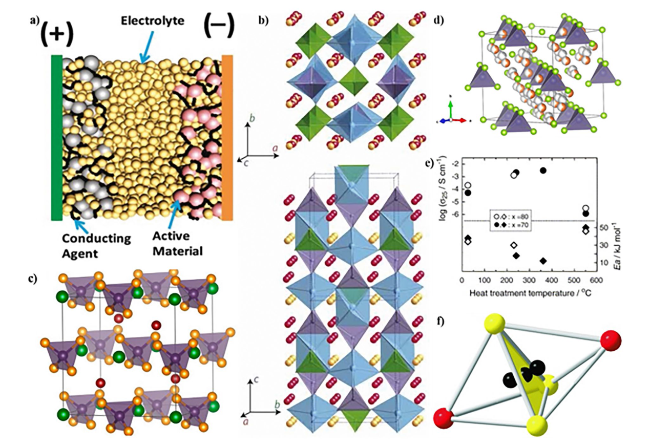
Fig. 3 a Schematic illustrating the structure of an all-solid-state battery. Copyright 2021, American Chemical Society [91]. b Crystal structure of Li10GeP2S12. Copyright 2011, Springer Nature Limited [54]. c Unit cell of the Li6PS5X (X = Cl, Br, I), PS43- units in the octahedral interstices. Copyright 2021, American Chemical Society [99]. d Cubic crystal structure of Ag8GeSe6 at 473 K. Copyright 2021, American Chemical Society [100]. e Heat treatment temperature dependences of the ambient temperature conductivities (σ25) and activation energies (Ea) for the xLi2S (100 − x) P2S5 (x = 70 and 80 mol%) glasses and glass-ceramics. Copyright 2006, Elsevier B.V. [101]. f A face-sharing S3I2 double tetrahedron. Copyright 2008, WILEY-VCH [102] |
3.1 Sulfide Based Solid State Electrolytes
3.1.1 Glasses
3.1.2 Crystalline Materials
3.1.2.1 Li-P-S Glassy Ceramics
3.1.2.2 Li6PS5X (X = Cl, Br, and I) Argyrodite
3.1.2.3 Thio-LISICONs
3.1.2.4 Li11−xM2−xP1+xS12 (M = Ge, Sn, and Si) Structures
3.2 Sulfide-Based Cathodes
3.2.1 Sulfur
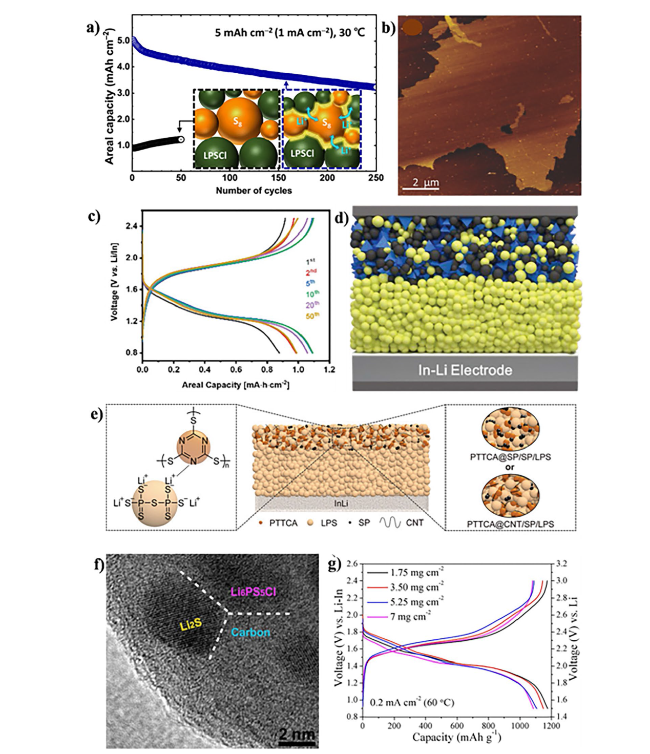
Fig. 4 a A scheme of inorganic Li-ion-conducting species (3Li+-PS4+n3- (n ≥ 0)) incorporated between S8 and the SSE of Li6PS5Cl (LPSCl) to enhance the ionic contact of S8. Copyright 2023, American Chemical Society [151]. b Atomic force microscopy (AFM) image of an amorphous rGO@S-40 composite on a Si substrate. Copyright 2017, WILEY-VCH [39]. c Electrochemical profile. Copyright 2020, WILEY-VCH [155]. d Schematic of the all-solid-state battery design for SnS nanocrystals. Copyright 2019, WILEY-VCH [156]. e Schematics of the ASSLSB with LPS electrolyte and poly (trithiocyanuric acid) PTTCA cathode (center), LPS-PTTCA interaction (left), and PTTCA@SP and PTTCA@CNT cathode topologies (right). Copyright 2021, WILEY-VCH [157]. f The high-resolution transmission electron microscopy (HRTEM) image of the as-obtained Li2S−Li6PS5Cl−C nanocomposite. Copyright 2016, American Chemical Society [158]. g Typical voltage profiles with areal Li2S loading from 1.75 to 7 mg cm−2. Copyright 2019, American Chemical Society [178] |
3.2.2 Metal Sulfide
3.2.3 Organic Sulfur
3.2.4 Lithium Sulfide
3.3 Anode Materials for All-Solid-State Lithium-Sulfur Batteries
3.3.1 Lithium Metal Anode
3.3.2 Lithium-Alloy Anode Materials
4 Challenges Redefined in All-Solid-State Lithium-Sulfur Batteries
4.1 Interface Stability
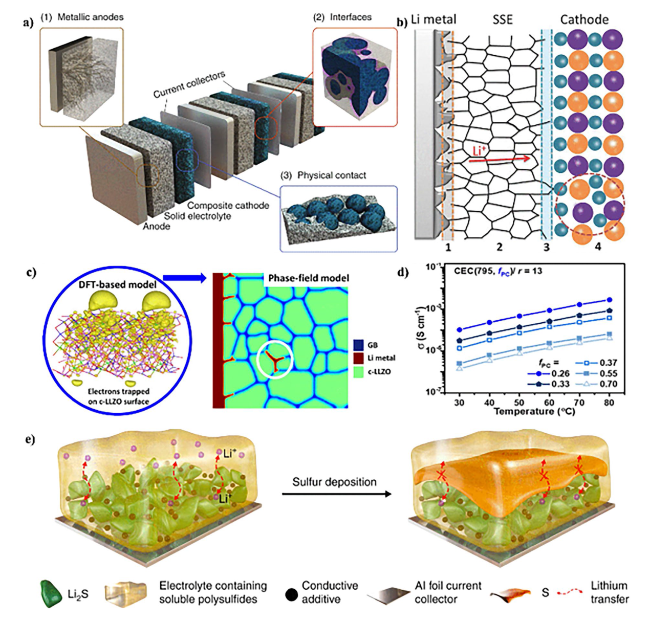
Fig. 5 a Schematic representation of a bipolar-stacked solid-state battery cell. Copyright 2020, WILEY-VCH [190]. b Schematic diagram of the main restrictions existing in ASSLSBs by using SSEs as the electrolyte. Copyright 2018, WILEY-VCH [104]. c Li dendrite growth morphology in polycrystalline LLZO. Copyright 2019, American Chemical Society [193]. d Varying PC volume fraction (fPC) at a fixed PEO (n = 795) and salt ratio (r = 13). Copyright 2022, American Chemical Society [196]. e Sulfur deposition and the resulting blocking cause the failure of a high-loading sulfur cathode. Copyright 2022, The Authors. Published by Springer Nature [199] |
4.2 Li Dendrite
4.3 Volume Expansion and Electrochemical Instabilities
4.4 Processing Challenges
4.5 Regulatory Approval and Standardization
5 Strategies to Accelerate the Commercialization of All-Solid-State Lithium-Sulfur Batteries
5.1 Enhance Performance
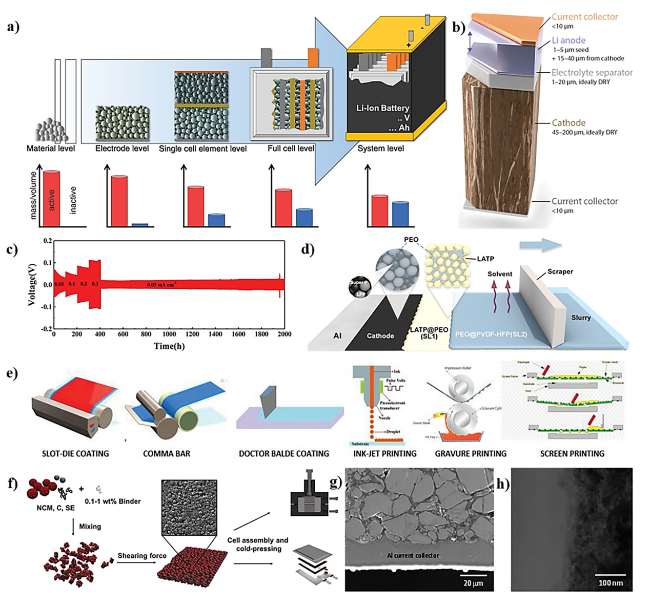
Fig. 6 a Technological level to be considered during battery development and a qualitative illustration of the respective active to inactive material ratio. Copyright 2021, WILEY-VCH [204]. b Schematic of an ideal high-energy solid-state battery stack including a thin cathode current collector, a thick cathode, a thin electrolyte separator, a thin Li anode that expands upon charging, and a thin anode current collector. Copyright 2021, American Chemical Society [208]. c Long cycling performance of Li/ZnO@LATP@ZnO/Li symmetric cells at various current densities. Copyright 2019, WILEY-VCH [209]. d Schematic illustration of the fabrication process of the IS-CDL. ©2020, American Chemical Society [210]. e Sketch of different wet coating techniques used for the fabrication of solid-state batteries. Copyright 2023, WILEY-VCH [211]. f Schematic diagram of DF-fabrication based on a dry premixing of NCM, C, SE, and PTFE binder followed by shearing force-induced film formation. Copyright 2019 Elsevier Ltd. [212]. g Cross-sectional FESEM image of the LPSCl-infiltrated LCO electrode, and h HRTEM image of the FIB-cross-sectioned LPSCl-infiltrated LCO electrode. Copyright 2021, American Chemical Society [213] |