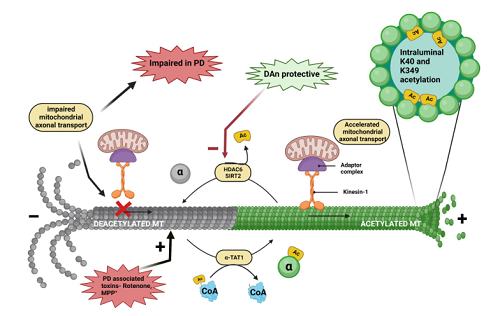
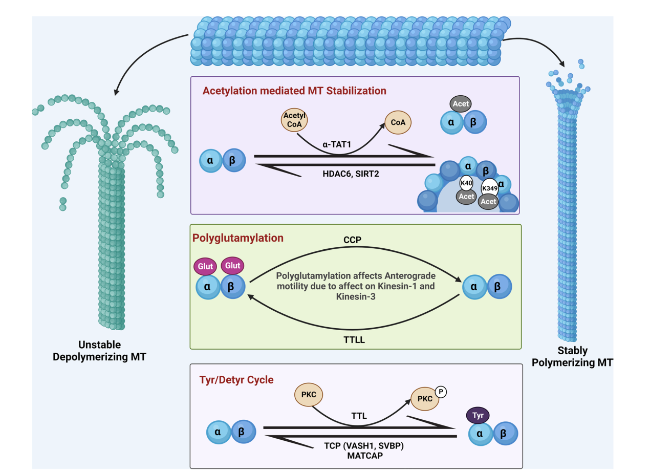
α-Tubulin acetyl transferase (α-TAT1) The enzymatic regulation of α-tubulin acetylation is achieved by the major acetylator α-TAT1 and two deacetylators, namely, HDAC6 and SIRT2. To date, at least 22 lysine acetyltransferases (KATs) have been identified in humans and they are grouped into three major families: the MYST family, the general control of amino acid synthesis 5 (GCN5)-related
N-acetyltransferases (GNAT) family, and the p300/CBP family. The prominently distinguished α-tubulin acetylator α-TAT1 belongs to the GNAT family of KATs, and in addition to this enzyme, MEC17 (
Caenorhabditis elegans protein mechanosensory abnormality 17), ARD1-NAT1 (arrest defective 1-aminoterminal, α-amino, acetyltransferase 1), the ELP complex (elongator protein complex) and GCN5 are also regarded as indirect candidate catalysts of α-tubulin K40 acetylation [
139,
140]. Several KATs are reported to be deregulated in PD and are implicated in pathological involvement at some level [
101]. A lack of detectable levels of K40 α-tubulin acetylation was observed in MEC17/α-TAT1-deficient mice. The deacetylation state was not increased by the other enzymes, such as ARD1-NAT1, ELP3 or GCN5, proving that they are not direct acetylators of α-tubulin and hence cannot compensate for the loss of α-TAT1 [
140]. Optimum levels of MEC-17 and ATAT-2 are needed for the temporal control of synaptic branching. Overexpression or loss of these enzymes causes a delay in synaptic branching and impaired synaptic formation in mechanosensory neurons of
Caenorhabditis elegans [
141]. Neuronal cells with defective elongators show a drastic reduction of acetylated α-tubulin. The ELP-3 catalytic domain of the elongator complex promotes acetylation and counteracts HDAC6-mediated deacetylation
in vitro. α-Tubulin is a target of the elongator complex [
139]. GCN5/KAT2A, although regarded as a histone acetyltransferase enzyme, has catalytic activity over MT as well. It modulates axonal outgrowth by regulating the proportion of acetylated tubulin [
142]. While histone acetyltransferase enzymes have been largely explored, there is a lack of structural and mechanistic data regarding α-TAT1. In comparison with the well elucidated KATs, α-TAT1 possesses a highly conserved co-substrate-binding domain that is unique in dual aspects, one being the active site and the other being the putative α-tubulin-binding site. A conserved glutamine residue was deduced as the propelling force behind the displayed catalytic activity of the enzyme [
143]. Friedmann et al. demonstrated that highly conserved aspartic acid and cysteine residues, D157 and C120, located on the active site of the enzyme, perform catalytic activity through the formation of a ternary complex. In comparison with GCN5 histone and Naa50p
N-amino acetyltransferases, α-TAT1 possesses a relatively wider substrate-binding groove of 20 Å [
144]. Whole protein docking was performed by Nung-Yu Hsu et al. to elucidate the different binding zones in the α-TAT1 crystal structure, and pharmacophore anchor models developed using SiMMap revealed three binding subpockets, namely, the S1 acetyl site, the S2 adenine site and the S3 diphosphate site. Validation of their model performed via BLAST across the α-TAT1 sequences of 14 species showed that the Q58, D157 and R158 residues were conserved at the S1 site. Mutations of D157E, R158A and Q58A induced a reduction/loss of the K40 acetyltransferase activity of α-TAT1 [
144,
145,
146]. Another highly conserved residue in the S2 site was identified as R132, and its mutation (R132A) decreased the enzymatic activity of α-TAT1 to 50% of wild-type activity [
145]. R132, H133 and G134 of the S3 diphosphate site are also highly conserved and are capable of forming hydrogen-bond interactions [
147]. Depletion of one of the cip/kip family members, p27
Kip1, is correlated with decreased α-tubulin acetylation. p27
Kip1 promotes the stabilization and regulation of α-TAT1 via binding to its highly conserved C-terminal domain, and its loss results in reduced levels of α-TAT1, leading to a subsequent decrease of α-tubulin acetylation and manifestation of axonal transport defects [
148]. α-TAT1 is hypothesized to act as a clock representing the life span of MTs [
122], and its deficiency causes an increase in the frequency of its mechanical breakage [
128]. By scanning the MT bidirectionally, Szyk et al. found that α-TAT1 acetylates the tubulin moieties without having any bias toward a particular end [
122]. Interestingly, α-TAT1 was found to have a self-acetylating property that regulates its function at α-tubulin. It has been established that α-TAT1 has specific affinity toward acetylating the α-tubulin of the MT and lacks acetylating activity toward histone protein substrates [
149]. The enzyme is considered to reach the acetylation site present on the luminal side, either by entering through the exposed ends of the MTs or by sneaking in through the crevices of structural irregularities in the MT framework [
150]. The precise molecular mechanism of luminal entry is an area under active investigation and requires further exploration. A recent study revealed that the active transport of α-TAT1-rich vesicles in axons is the predominant driving force for axonal MT acetylation. It was also demonstrated that precise bidirectional vesicular transport requires proper α-tubulin acetylation, mediated via α-TAT1, and functional loss of this enzyme leads to impaired axonal transport in neurons [
151]. Increased α-TAT1-mediated MT acetylation was found to be the cause of breast cancer cell metastasis [
152], while knockout of α-TAT1 in colon cancer cells inhibits their proliferation and invasive migration potential [
153]. In the case of neurodegenerative conditions such as AD and PD, an increase in MT acetylation was found to benefit MT dynamics and enhance the binding of MAPs such as α-syn and tau with tubulin tracks [
78,
154]. MTs are promising therapeutic targets for neurodegenerative diseases. While a small number of α-TAT1 inhibitors exist, there are no moieties that can activate α-TAT1 hitherto [
155]. Hence, an in-depth comprehensive exploration regarding the molecular basis of α-TAT1-mediated MT acetylation could potentially open the doorway for designing small-molecule modulators that enhance or reduce the acetylation phenomena of MT tracks.