Highlights
1 Introduction
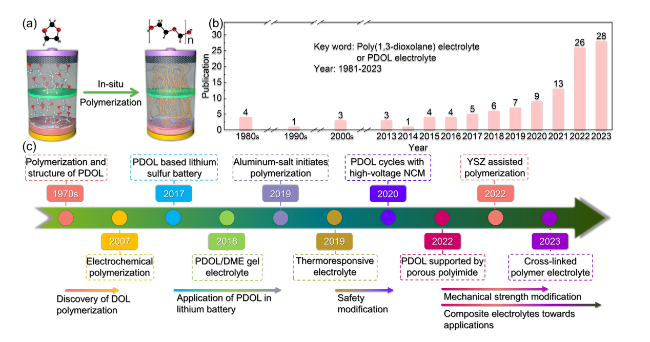
Fig. 1 Overview of research progress on PDOL polymer. a Schematic diagram of in-situ polymerization of DOL monomer inside the battery. b Number of publications related to PDOL electrolyte in each year (Keyword: Poly(1,3-dioxolane) electrolyte or PDOL electrolyte; Data from Web of Science). c Development process of PDOL polymer: The polymerization of DOL and the structure of PDOL began to be studied (1968-1972) [60,61,62], Current is used to initiate DOL polymerization (2007) [63], PDOL inhibits the shuttle effect of polysulfides in lithium-sulfur batteries (2017) [56], PDOL/DME gel electrolyte enabled stable cycle with LiFePO4 cathode (2018) [58], Initiating DOL polymerization with anhydrous participation of trace aluminum salts (2019) [64], PDOL/PLAS thermoresponsive electrolyte prevents thermal runaway (2019) [65], Realizing normal cycling of PDOL initiated with the assistance of AlF3 and high-voltage NCM cathode (2020) [66], Porous polyimide supported PDOL enhances the mechanical strength of the electrolyte (2022) [67], PDOL added by YSZ enables long life cycling at high voltage (2022) [68], PDOL-based cross-linked electrolyte enhances the stability of lithium metal (2023) [69] |
2 Fundamental Mechanism of DOL Polymerization
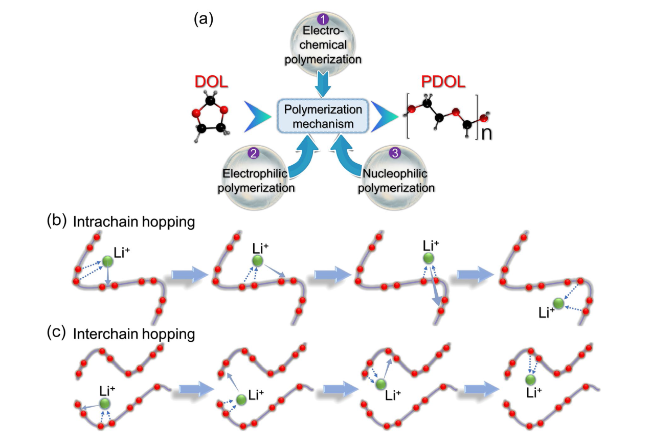
Fig. 2 Polymerization mechanism and Li+ conduction mechanism of DOL. a Three polymerization mechanisms of DOL: electrochemical polymerization, electrophilic polymerization, and nucleophilic polymerization [71]. Schematic diagram of b intrachain conduction mechanism and c interchain conduction mechanism for Li.+ in PDOL [72] |
2.1 Electrochemical Polymerization of DOL
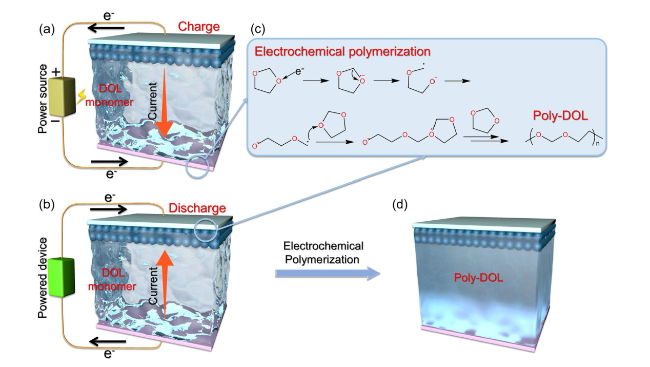
Fig. 3 Applications of current-initiated DOL polymerization. Schematic diagram of electronic and current movements during a charging and b discharging processes. c Electrochemical polymerization mechanism of DOL. d Battery state after current-initiated DOL polymerization [45] |
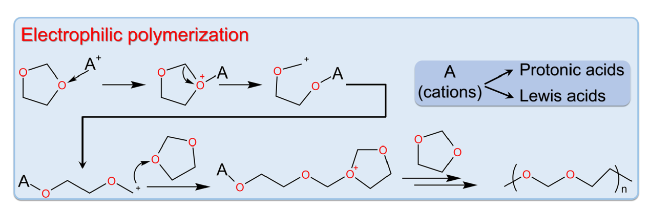
2.2 Electrophilic Polymerization of DOL
Fig. 4 Electrophilic polymerization mechanism of DOL [71] |
Table 1 Classification of different initiators and performance comparison of the PDOL electrolyte initiated by them |
| Type of initiator | Initiator | Initiation factor | Initiator content | DOL conversion rate (%) | PDOL molecular weight | Electrolyte state | Ionic conductivity (mS cm−1) | Oxidation potential (V) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Lithium salt | LiPF6 | Protonic acid | 2.0 M | 91 | ~ 53 000 (Mn) | Gel | 3.8 | 4.6 | [58] |
| LiPF6 | Protonic acid | 1.0 wt% | 98.5 | 23 588 (Mw) | Solid | 0.28 | 5.2 | [68] | |
| LiDFOB | Protonic acid | 0.3 M | 90 | / | Gel | 0.39 | 5.1 | [75] | |
| LiBF4 | Protonic acid | 0.2 M | 81.2 | - | Solid | 0.3 | 4.9 | [76] | |
| LiFSI | Protonic acid | 3.5 M | 75 | - | Gel | 7.9 | 4.7 | [77] | |
| Aluminum salt | Al(OTf)3 | Lewis acid | 0.5 mM | 86 | 15 000 (Mn) | Solid | 1.1 | 5.0 | [64] |
| Al(OTf)3 + AlF3 | Lewis acid | 0.5 mM + 0.3 M | 12 | 18 000 (Mw) | Solid | 1.8 | 4.7 | [66] | |
| AlI3 | Lewis acid | 600 ppm | 84 | - | Gel | - | - | [57] | |
| Tin salt | Sn(OTf)2 | Lewis acid | 2.0 mM | 90 | 12 417 (Mw) | Gel | 6.2 × 10-2 | 4.9 | [78] |
| SnF2 | Lewis acid | 1.5 wt% | 92 | 29 552 (Mw) | Solid | 1.7 × 10-2 | 5.0 | [79] | |
| Other salts | ZnCl2 | Lewis acid | 25 wt% | - | 51 798 (Mn) | - | - | - | [50] |
| Sc(OTf)3 | Lewis acid | 1.0 mM | 84 | 48 963 (Mn) | Solid | 8.7 × 10-2 | ~ 5.0 | [80] | |
| Mg(OTf)2 | Lewis acid | 1.0 wt% | 79.9 | ~ 57 000 (Mn) | Solid | 0.5 | 4.3 | [81] | |
| Acidized materials | Acidized Al2O3 | Protonic acid | 4.0 wt% | 89.6 | 32 000 (Mn) | Gel | 3.37 | 4.5 | [82] |
| Acidized CNT paper | Protonic acid | - | - | - | Gel | - | - | [56] | |
| Organic materials | TB | Protonic acid | 3.0 wt% | 89 | - | Solid | 1.16 | 4.8 | [83] |
| S(C2H4O4) | Lewis acid | 0.3 M | 88.2 | 5 391 (Mn) | Solid | 0.38 | 4.7 | [84] | |
| HFiP | Nucleophilic | 0.5 wt% | 67 | - | Gel | 1.6 | 4.7 | [85] |
2.2.1 Polymerization Initiated by Lithium Salt
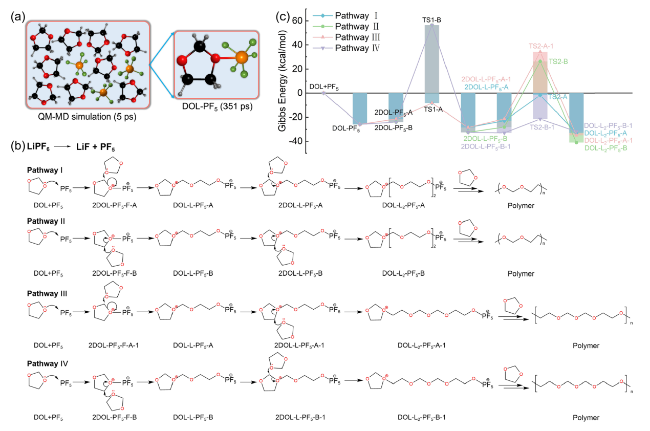
Fig. 5 Schematic diagram of LiPF6-initiated DOL polymerization process. a Structure of DOL-PF5 obtained from the 3ps QM-MD simulation of PF5-DOL system. b Four possible paths of DOL ring-opening polymerization under the action of PF5 and c reaction Gibbs free energy [86] |
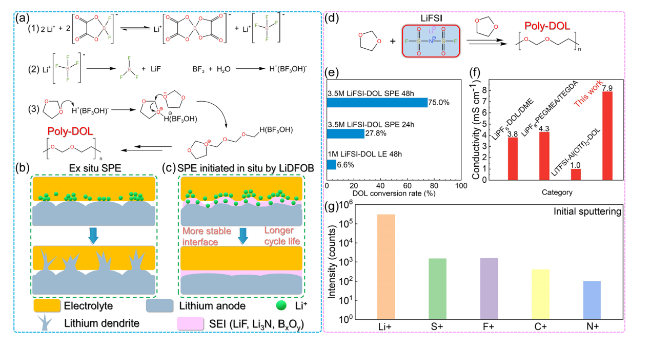
Fig. 6 LiDFOB (LiBF4) or LiFSI initiated DOL polymerization. a Schematic illustration of the LiDFOB initiated DOL ring-opening polymerization process. b Interphase evolution of ordinary non-in situ polymer electrolytes leads to incomplete interface contact, resulting in uneven ion transport and rapid growth of lithium dendrites [87]. c LiDFOB in-situ initiated polymerization of PDOL electrolyte generates SEI rich in boron oxide compounds at the electrolyte/lithium anode interface, which guides ion uniform deposition, resulting in a more stable interphase and longer cycle life. d LiFSI initiates DOL ring-opening polymerization process. e DOL polymerization conversion rate at different LiFSI content and standing time. f Comparison of ionic conductivity of PDOL gel electrolyte initiated by LiFSI with other PDOL electrolytes. g TOF-SIMS of C + , F + , S + , N + and Li + species on the lithium surface after cycling in the gel electrolyte: species concentration at initial sputtering [77] |
2.2.2 Polymerization Initiated by Aluminum Salt
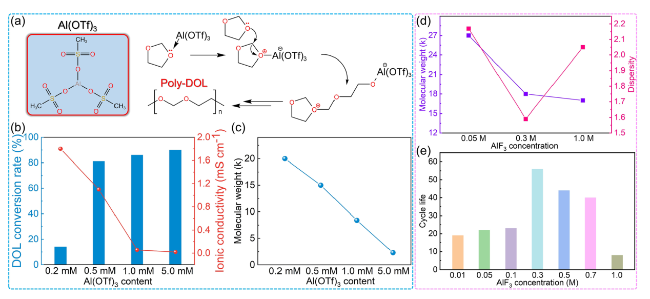
Fig. 7 Aluminum salt initiates DOL polymerization. a Mechanism of DOL polymerization initiated by Al(OTf)3. b DOL polymerization conversion rate and c molecular weight of PDOL initiated by different aluminum salt initiators content [64]. After adding different contents of AlF3, d the molecular weight and dispersity of PDOL, and e cycle life of PDOL [66] |
2.2.3 Polymerization Initiated by Other Typical Initiators
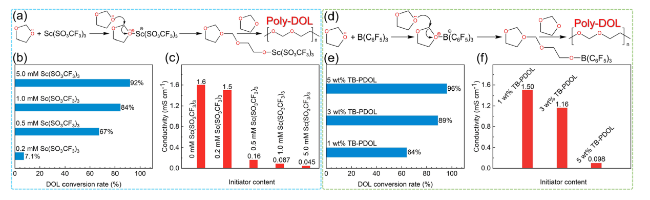
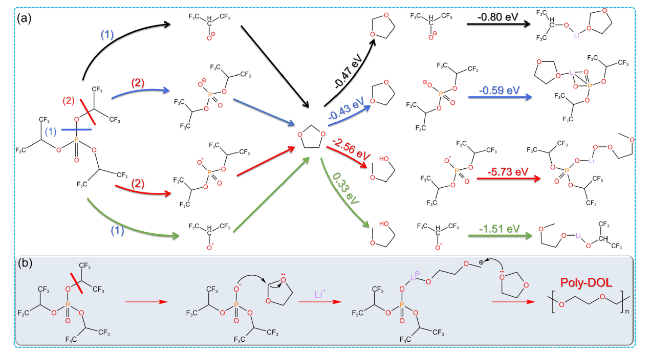
Fig. 8 Scandium salt and TB initiate DOL polymerization. a Mechanism of DOL polymerization initiated by Sc(SO3CF3)3. b DOL polymerization conversion and c ionic conductivity of PDOL electrolyte initiated with different initiator concentrations [92]. d TB initiates the DOL polymerization process. e DOL polymerization conversion rate and f room temperature ionic conductivity initiated by different TB contents [83] |
2.3 Nucleophilic Polymerization of DOL
Fig. 9 Nucleophilic polymerization mechanism of DOL. a Some free radicals generated from the decomposition of HFiP and their free energy for ring-opening reactions with DOL. b Schematic diagram of HFiP initiated nucleophilic polymerization of DOL [85] |
3 Interface of PDOL Electrolyte
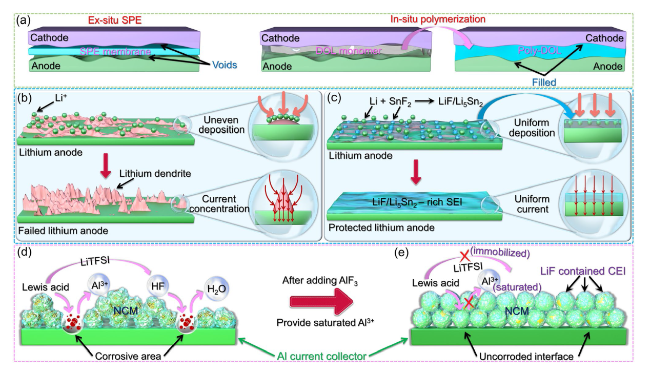
Fig. 10 Contact mode and interface optimization between PDOL electrolyte and solid-state electrode. a Differences in interface contact modes between electrolytes and solid-state electrodes in ex-situ polymerization and in-situ polymerization [64]. b The uneven deposition of lithium ions on common lithium metal anodes leads to rapid growth of lithium dendrites: in areas where lithium ions are concentrated and deposited, it will further guide the aggregation of ions and currents, leading to the growth of dendrites. c In the SnF2-initiated PDOL electrolyte, the initiator undergoes a reaction with lithium to generate Li-Sn alloy, and it then forms a robust LiF/Li5Sn2-rich SEI incorporating LiF. The SEI facilitates the homogeneous deposition of lithium ions and the even distribution of current, providing protection to the lithium metal anode [79]. d Mechanism diagram of proton corrosion of aluminum current collector caused by Lewis acid interface reaction. e Mechanism diagram of aluminum current collector corrosion inhibition after adding AlF3 [66] |
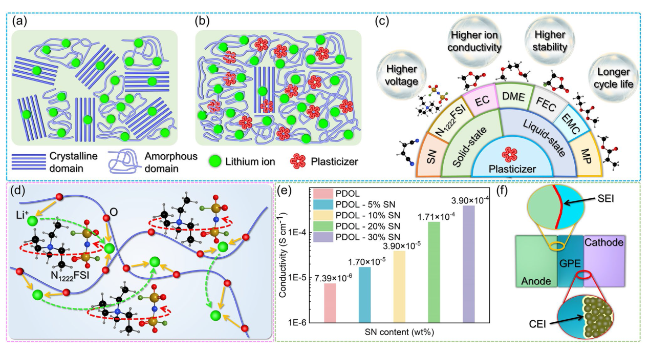
Fig. 11 Improvement of PDOL electrolyte performance by plasticizers. a PDOL polymers without plasticizers possess a significant amount of crystalline domains in their structure, which hinders the mobility of polymer chains. b The addition of plasticizers to PDOL increases the amorphous domains, facilitating chain segment mobility and resulting in enhanced ion conductivity. c The reported types of plasticizers and their enhancements on the performance of PDOL polymers. d The internal rotational motion of N1222FSI leads to orientation disorder and the generation of lattice vacancies in PDOL, thereby enhancing ion conductivity [87]. e The effect of different SN contents on the ion conductivity of PDOL GPE. f The CEI and SEI gradually formed during the cycling process of GPE protect the cathode and anode, respectively [75] |
4 Composites of PDOL Electrolyte
4.1 Plasticizers
4.2 Support Skeleton
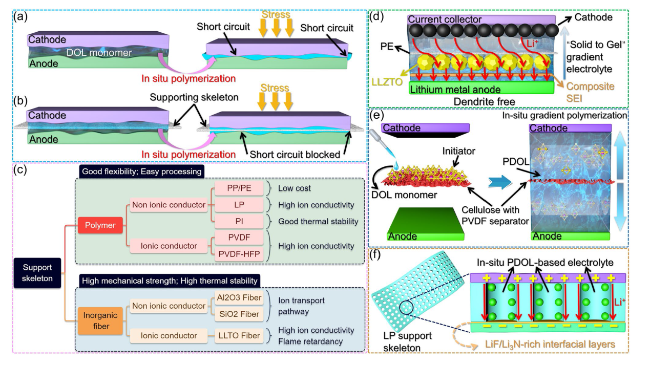
Fig. 12 Function and classification of support skeleton. a In the absence of a support skeleton, the electrode may come into contact and experience short circuits under external pressure during the in-situ polymerization process of DOL. b The presence of support skeletons effectively serves as a mechanical barrier, preventing electrode contact and mitigating the risk of short circuits during the in-situ polymerization process. c The types of support skeletons and their corresponding advantages, they can be broadly categorized into two main groups: polymer-based and inorganic-based materials. d Schematic diagram of the battery structure after DOL gradient polymerization initiated by modified PE, the densely polymerized PDOL solid electrolyte adjacent to the anode serves as a protective barrier for the lithium metal, while the partially polymerized PDOL gel electrolyte in close proximity to the porous cathode effectively permeates and infiltrates the electrode [131]. e Schematic diagram of bidirectional gradient polymerization of DOL on modified cellulose paper [132]. f Schematic diagram of the interphase layer between electrolyte and LP to enhance ion conductivity [76] |
4.2.1 Polymer Support Skeleton
4.2.2 Inorganic Fibers Support Skeleton
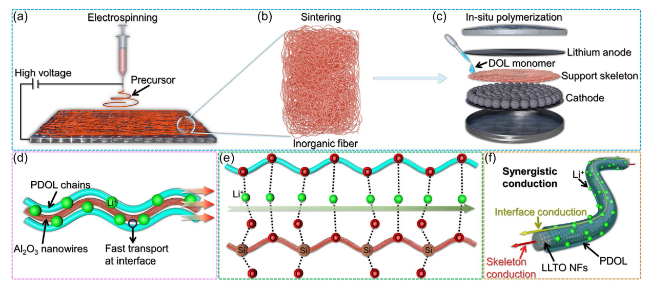
Fig. 13 Inorganic fiber materials as PDOL support skeleton. a The precursor of inorganic materials is electrospun to prepare a fiber network at high voltage. b Inorganic material precursors are sintered to form inorganic fiber support skeletons. c Structural diagram of a battery supported by an inorganic fiber skeleton through in-situ polymerization. d The interface between Al2O3 nanowires and PDOL polymer segments constructs a high-speed ion transport channel, accelerating Li+ transport [92]. e The oxygen atoms of PDOL and the Si-O bonds of SiO2 collectively create a highly efficient pathway for Li+ migration [139]. f LLTO NFs possess Li+ transport capability, while the interface between LLTO NFs and PDOL also exhibits high-speed Li+ transport capability, the synergistic conduction of both components enhances the overall ionic conductivity of the electrolyte [80] |
4.3 Inorganic Fillers
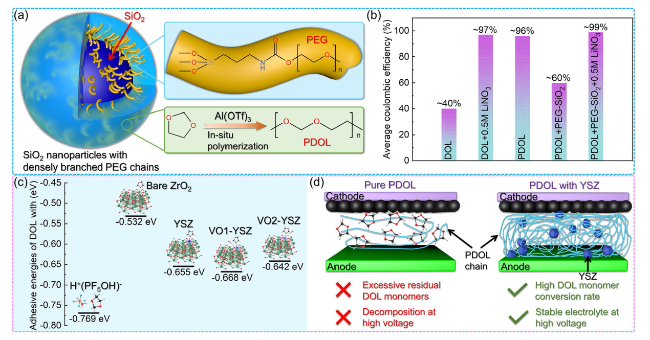
Fig. 14 Inorganic materials enhance the performance of PDOL. a Schematic diagram of PEG grafting on the surface of SiO2 and co-crystallization with PDOL. b Average coulombic efficiency for 200 cycles of Li//Cu battery assembled with PDOL-SiO2 composite electrolyte [151]. c DFT calculated ring-opening binding energy of DOL monomer with initiator, ZrO2, zirconium atom on YSZ and oxygen vacancy on YSZ. d The auxiliary activation of YSZ substantially enhances the monomer conversion rate of DOL, resulting in a significant improvement in the high voltage capability of the electrolyte, this enhancement enables the attainment of a long service life that aligns with high voltage cathode materials [68] |
5 PDOL-Based Electrolytes for Solid-State Lithium Batteries
5.1 Cycle Performance at Ambient Temperature
Table 2 Cyclic performance of PDOL polymer electrolyte matched with different cathodes |
| Battery type | Cathode materials | Initiator | Additive | Voltage range (V) | Initial specific capacity (mAh g−1) | Rate (C) | Cycle number | Capacity retention (%) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Lithium-sulfur batteries | Sulfur | ACNTP | - | 1.7-3.0 | 683 | 1.0 | 400 | 66.5 | [56] |
| Sulfur | LiPF6 | DME | 1.8-3.0 | 1005 | 0.5 | 500 | 73.7 | [58] | |
| Sulfur | LiPF6 | DME/Al2O3 | 1.8-3.0 | 1102 | 0.1 | 200 | 73.0 | [59] | |
| Sulfur | LiFSI | DME | 1.7-2.8 | 910 | 0.2 | 300 | 75.5 | [77] | |
| Sulfur | HFiP | DME | 1.8-2.8 | 1025 | 0.5 | 500 | 68.1 | [85] | |
| Sulfur | LiPF6 | DME | 1.7-2.8 | 870 | 0.1 | 500 | 45.0 | [163] | |
| Lithium-ion batteries | LFP | LiPF6 | DME | 2.5-4.0 | 137 | 0.5 | 700 | 95.6 | [58] |
| LFP | LiDFOB | SN | 2.5-4.0 | 127 | 1.0 | 1 000 | 83.55 | [75] | |
| LFP | LiFSI | DME | 2.5-3.8 | 147 | 1.0 | 500 | 69.3 | [77] | |
| LFP | Sc(OTf)3 | - | 2.5-4.0 | 155 | 0.5 | 200 | 83.5 | [91] | |
| LFP | Al(OTf)3 | PDA | 3-3.75 | 145 | 1.0 | 200 | 87.1 | [137] | |
| LFP | Y(OTf)3 | LLZTO | 2.8-3.8 | 135 | 0.2 | 200 | ~ 70.0 | [142] | |
| LFP | LiPF6 | PI/DME | 2.5-3.7 | 152 | 0.5 | 200 | 91.8 | [67] | |
| LFP | Mg(OTf)2 | FEC | 2.8-4.2 | 127 | 1.0 | 2 000 | 94.0 | [81] | |
| LFP | LiDFOB | FEC/SN | 2.8-4.3 | 142 | 2.0 | 1 000 | 95.0 | [123] | |
| NCM622 | LiPF6 | DME | 2.8-4.3 | 165 | 0.1 | 100 | 90.9 | [58] | |
| NCM622 | Al(OTf)3 | AlF3 | 2.8-4.2 | 160 | 0.5 | 30 | 80 | [66] | |
| NCM622 | LiPF6 | - | 2.8-4.3 | 138 | 0.5 | 300 | 85 | [129] | |
| NCM622 | LiPF6 | YSZ | 2.8-4.3 | 165 | 0.5 | 800 | 74 | [68] | |
| NCM523 | LiFSI | - | 3.0-4.3 | 136 | 0.1 | 100 | 94 | [89] | |
| NCM811 | LiDFOB | FEC/SN | 2.8-4.3 | 145 | 0.2 | 100 | 70.6 | [123] | |
| NCM83 | S(C2H4O4) | - | 2.8-4.5 | 205 | 0.5 | 100 | 89.5 | [84] | |
| LCO | LiDFOB | SN | 2.8-4.3 | 140 | 0.1 | 60 | 57.1 | [75] | |
| LMFP | LiBF4 | TTE | 3.0-4.3 | 130 | 0.2 | 100 | ~ 90 | [76] | |
| Sodium-ion batteries | NVP | Al(OTf)3 | FEC | 2.0-3.8 | 99 | 5.0 | 2 000 | 93.6 | [125] |
5.2 Safety and Thermal Stability
5.3 Low-Temperature Performance
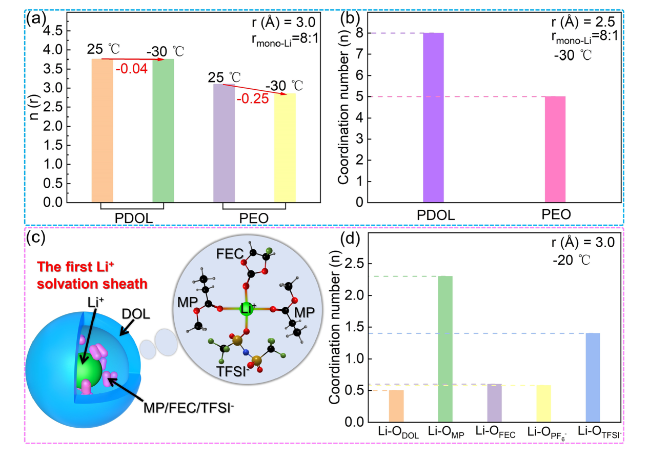
Fig. 15 Low temperature performance of PDOL. a Li-O integrated RDF of PDOL and PEO (rmono-Li = 8:1). b Local coordination number of Li+ and O atoms of PDOL and PEO at −30 °C (rmono-Li = 8:1) [166]. c The first Li+ solvation sheath and snapshot of QSPE from MD simulation for QSPE at -20 °C. d The coordination numbers of Li+ in the first solvation shell (3 Å) in QSPE at − 20 °C [128] |
5.4 Instability of PDOL Electrolyte
6 Perspectives and Conclusions
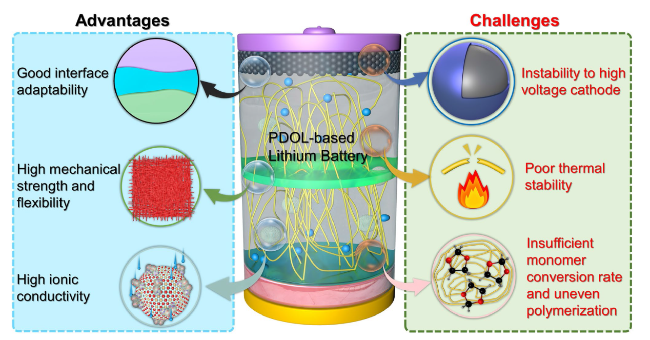
Fig. 16 Advantages of PDOL polymer electrolytes and existing challenges in practical applications |