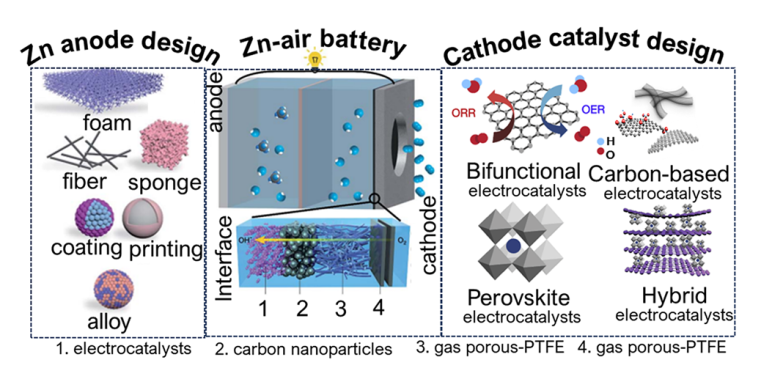
Highlights
1 Introduction
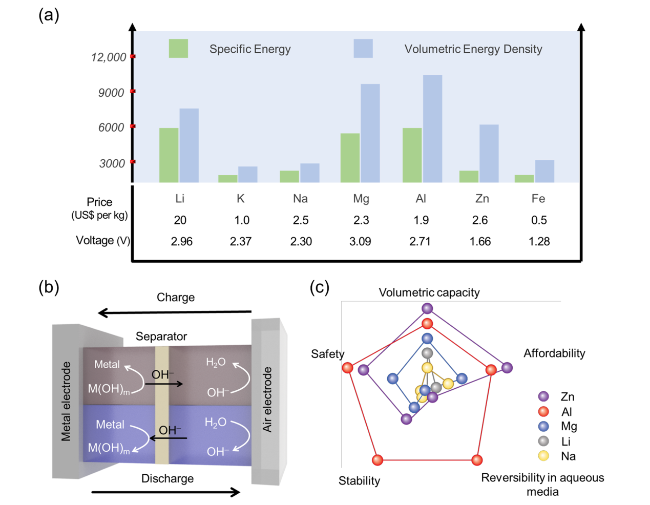
Fig. 1 a Theoretical specific energies, volumetric energy densities, nominal cell voltages, and properties for various metal anodes, b schematic diagram of a ZAB, and c comparison of the theoretical specific energies, safety, stability, reversibility in aqueous media, and affordability of metal-air batteries [22,23] |
2 Fundamentals, Working Principle, and Mechanism of Rechargeable ZABs
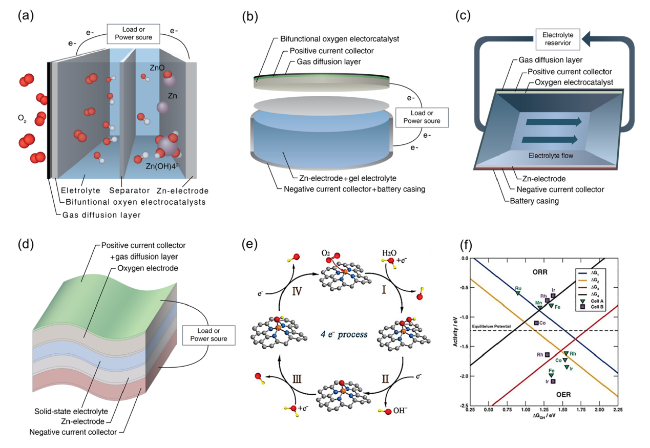
Fig. 2 Four common rechargeable ZAB configurations: a a planar battery with an aqueous electrolyte, b a planar battery with a gel electrolyte, c a flow battery, and d a flexible battery. Reproduced with permission from [28]. e Illustration of intermediates in the ORR and OER processes. f Theoretical ORR and OER volcano plots of overpotential based on the scaling relationships [50]. Copyright 2023, Wiley |
2.1 Mechanisms of ORR
2.2 Mechanisms of OER
3 Challenges and Progress on Basic Components of ZABs
3.1 Anode Materials
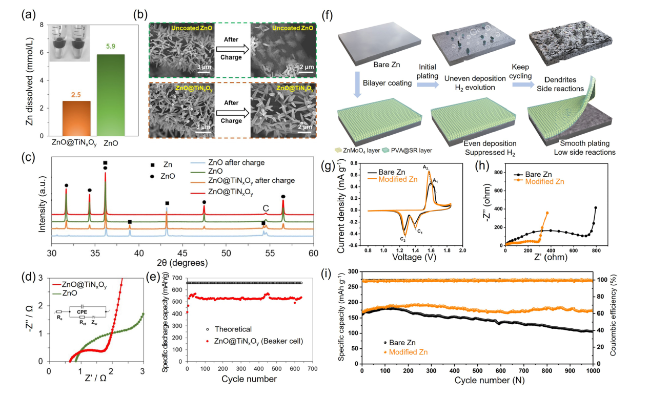
Fig. 3 a Zn dissolution (mmol L−1) in a 4 M KOH solution. b Scanning electron microscopy (SEM) micrographs of uncoated ZnO and ZnO@TiNxOy anode before and after the charging process. c X-ray diffraction patterns for ZnO nanorods and a ZnO@TiNxOy anode before and after the charging process. d Electrochemical impedance spectroscopy (EIS) results and the related equivalent circuit for uncoated ZnO and a ZnO@TiNxOy nanorod anode. Reproduced with permission from [64]. e Cycling performance of ZnO@TiNxOy nanorod anode (2 mg cm−2) with 200 cycles ALD at 0.5 C charge and 2 C discharge rates in beaker cell with 10 mL ZnO saturated 4 M KOH electrolyte. The cutoff voltages are 1.4/2 V. One dot every five data points. Produced with permission from [64]. f Cycling performance of bare Zn and PVA@SR-ZnMoO4 SEI-like structure coating modified Zn. g CV curves at 0.1 mV s−1. h EIS curves before cycling. i Long-term cycling performance at 1.0 A g.−1. Produced with permission from [65] |
Table 1 Zn anodes used for the construction of ZABs |
| Structural assessment and electrolyte | Anode | Specific capacity (mAh g−1) | Discharge/charge voltage gap (V) | Cyclic performance | References |
|---|---|---|---|---|---|
| Hard carbon (HC), 1 M Zn(OTf)2 | rGO-SnCu/Zn | - | ~ 0.7 | 1200 h @0.25 mA cm−2 | [76] |
| Zn surface modification, 2 M ZnSO4 + 0.2 M MnSO4 | Zn-AgNWs | 243.9 | 0.76 | 800 cycles@0.6 A g−1 | [61] |
| Bottom cell, 2 M Zn(SO4)2 + 0.1 M MnSO4 | Zn@ZrP | 132.4 | - | 780 h @0.5 mA cm−2 | [77] |
| 3D zinc anode, 1 M ZnSO4 + 1 M KCl | Ag-modified Cu foam | 676 | 1.02 | 80 cycles, 2 h @25 mA cm−2 | [78] |
| Coin cell, 3 M ZnSO4 + 0.1 M MnSO4 | Zn-Sb3P2O14 | 111.7 | - | 450 h @10 mA cm−2 | [79] |
| 3D porous framework, 6 M KOH | Zn anode | 812 | 0.63 | 33 cycles @5 mA cm−2 | [80] |
| Coin cell, 6 M KOH and 0.2 M Zn(AC)2 | Ti3C2Tx-protected Zn | - | 0.6 | 400 cycles @5 mA cm−2 | [81] |
| Carbon cloth (CC) cathode, 2 M ZnSO4, | Zn@ZIF8 | 158 | - | 750 h @1.0 mA cm−2 | [82] |
| Layered structure, 6 M KOH + 0.2 M Zn(Ac)2 + S ZnO aqueous solution | Tin-coated copper foam (CF@Sn) | 800 | - | 5220 h @10 mA cm−2 | [83] |
3.2 Electrolytes
Table 2 Summary of recently described electrolytes for ZABs |
| Electrolyte composition (type) | Electrode materials | Specific capacity (mAh gzn−1) | Power density | Cyclic performance | References |
|---|---|---|---|---|---|
| 6 M KOH + 0.2 M zinc Acetate (alkaline) | Zinc plate//Co-Co3O4@NAC@NF | 721 @10 mA cm−2 | 164 mW cm−2 @0.63 V | 35 h@10 mA cm−2 | [97] |
| 6 M KOH + 0.2 M zinc Acetate (alkaline) | Zinc foil//Co3O4−x@CP | 800 @5 mA cm−2 | 122 mW cm−2 @230 mA cm−2 | 150 h@5 mA cm−2 | [98] |
| 7 M KOH + 5-20% v/v DMSO (alkaline) | Zinc granules//MnO2@NF | 550 @10 mA cm−2 | 130 mW cm−2 @150 mA cm−2 | 600 cycles@discharge @75 mA cm−2 | [99] |
| 6 M KOH + 0.2 M ZnCl2 (alkaline) | Zinc plate//Pt-SCFP@CC | 781 @10 mA cm−2 | 122 mW cm−2 @214 mA cm−2 | 80 h@5 mA cm−2 | [100] |
| 8 M KOH + 0-50% v/v Ethanol (alkaline) | Zinc granules//MnO2@NF | 470 @25 mA cm−2 | 32 mW cm−2 @30 mA cm−2 | - | [101] |
| 6.0 M KOH + 0.2 M Zn(OAc)2 polyacrylamide/montmorillonite (PAM/MMT) (GPE) | ZAB-Mn-SAC | 631 | 30 mW cm−2 | 29 h@2.0 mA cm−2 | [102] |
| KOH + DMSO + poly(2-acrylamido-2-methylpropanesulfonic acid)/polyacrylamide (PAMPS/PAAm) (GPE) | Organohydrogel electrolyte (OHE)-based ZAB | 562 | 21.8 mW cm−2 | 45 h@2.0 mA cm−2 | [103] |
| Polyacrylic acid (PAA) + polyacrylamide (PAM) in glycerol (GPE) | Zn anode// carbon cloth cathode containing Pt/C and RuO2 | 506.2 | 8.2 mW cm−2 | 10 h @1.0 mA cm−2 | [104] |
| PAM-CNF/KOH/KI (GPE) | cable-type ZAB | 743 | 10 mW cm−2 | 45 h @2 mA cm−2 | [105] |
| poly(2-acrylamido-2-methylpropanesulfonic acid potassium salt) (PAMPS-K) + methyl cellulose (MC) (GPE) | Zinc plate // Co3O4 nanoparticle/CC | 754.2 | 54.2 mW cm−2 | 24 h @1 mA cm−2 | [106] |
NAC N-doped active carbon, NF Nickel foam, CP Carbon paper, CC Carbon cloth, DMSO Dimethyl sulfoxide |
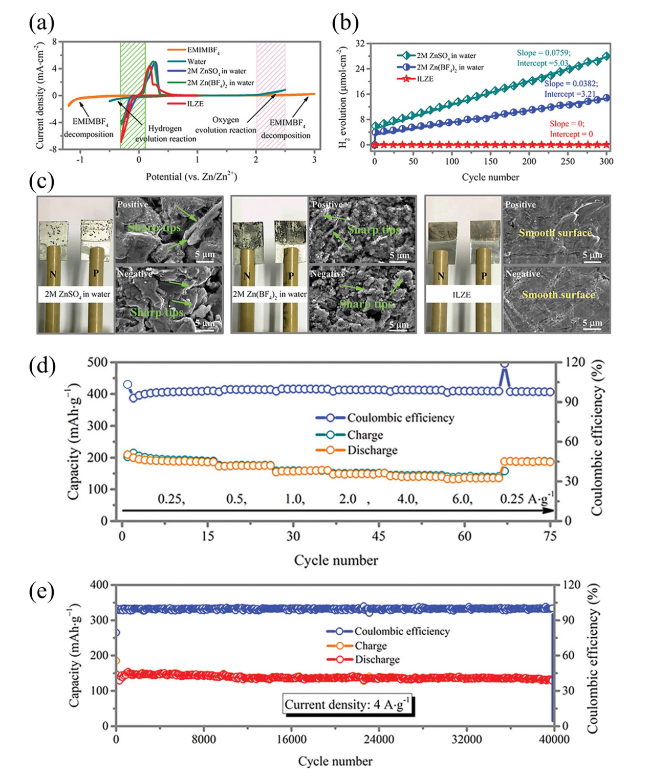
Fig. 4 a Three-electrode configuration-based cyclic voltammetry (CV) curves for prepared Zn-ion battery in various electrolytes, b H2 evolution according to the number of cycles measured at 0.5 mA cm−2, c surface morphology of Zn foil after 300 cycles measured at 0.5 mA cm−2, d cyclic stability and coulombic efficiency at an applied current density of 4 A g−1 for 40,000 cycles, and e rate performance. Reproduced with permission from [90] |
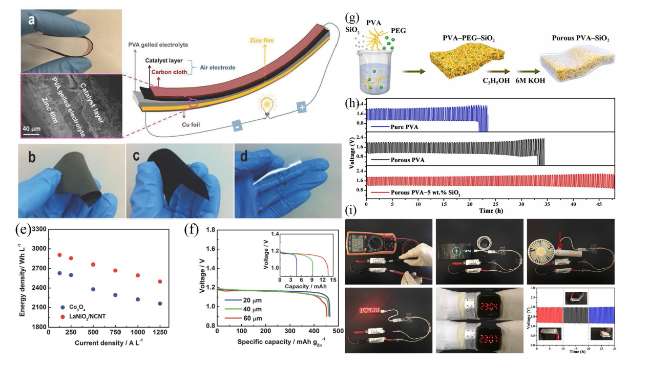
Fig. 5 a Flexible, solid-state rechargeable ZAB illustrated using a photograph of its bending ability (top), a cross-sectional SEM image (bottom), and a schematic diagram of its structure (right). b Optical photograph of the freestanding Zn electrode film. c Optical photograph of the bifunctional catalytic air electrode using LaNiO3/NCNT. d Optical photograph of porous PVA-gelled electrolyte membrane. e Comparative analysis of the energy and current density for an all-solid-state ZAB prepared using the bifunctional catalyst Co3O4 and a LaNiO3/NCNT-based air electrode. f Specific capacity curves for the prepared ZABs as a function of the Zn film thickness. Reproduced with permission from [95]. g Scheme displaying the preparation of flexible a ZAB using a porous PVA nanocomposite-based GPE. h GCD curves for ZABs using different electrolytes at 3 mA cm−3 and 20 min per cycle. i Assembled ZABs used as a power source for various electronic devices. Reproduced with permission from [96] |
3.3 Separators
3.4 Air Electrodes
4 Design of Air Catalysts
4.1 Bifunctional Oxygen Electrocatalysts
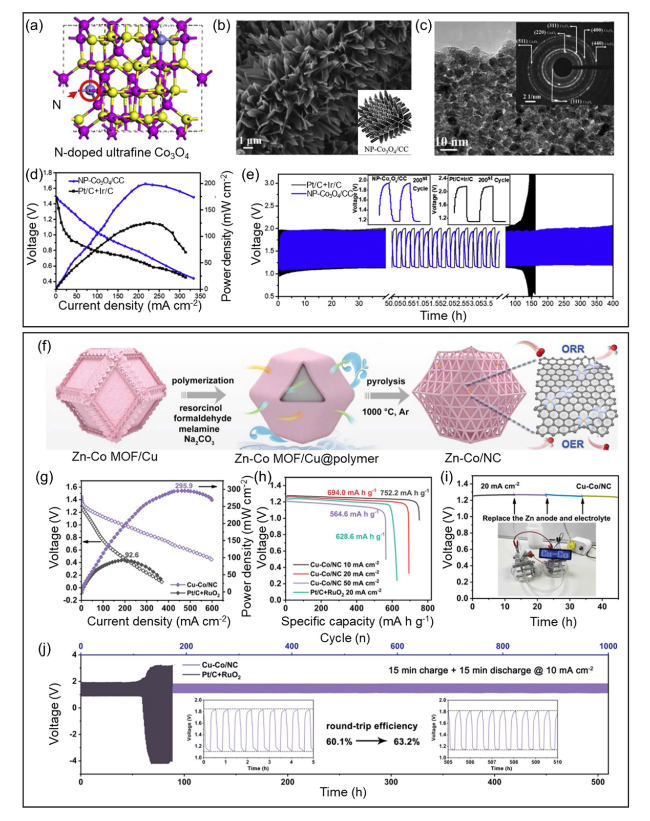
Fig. 6 a Scheme showing the preparation of NP-Co3O4/CC and associated reaction mechanisms. b SEM micrographs for NP-Co3O4/CC. c HRTEM images for NP-Co3O4. d Battery voltage and power density and e Galvanostatic discharge-charge cycling curves at 5 mA cm.−2 of aqueous rechargeable ZABs with the NP-Co3O4/CC and Pt/C + Ir/C catalyst as the air electrode, respectively. Produced with permission from [143]. f Synthetic scheme of Cu-Co/NC. g Discharge polarization curves and the corresponding power densities. h Specific capacities of zinc-air batteries at different discharge current densities. i Long-term durability of primary zinc-air battery with Cu-Co/NC catalyst. j Galvanostatic discharge/charge cycling curves (the inset shows the round-trip efficiency of zinc-air battery at first 10 cycles and last 10 cycles). Produced with permission from [144] |
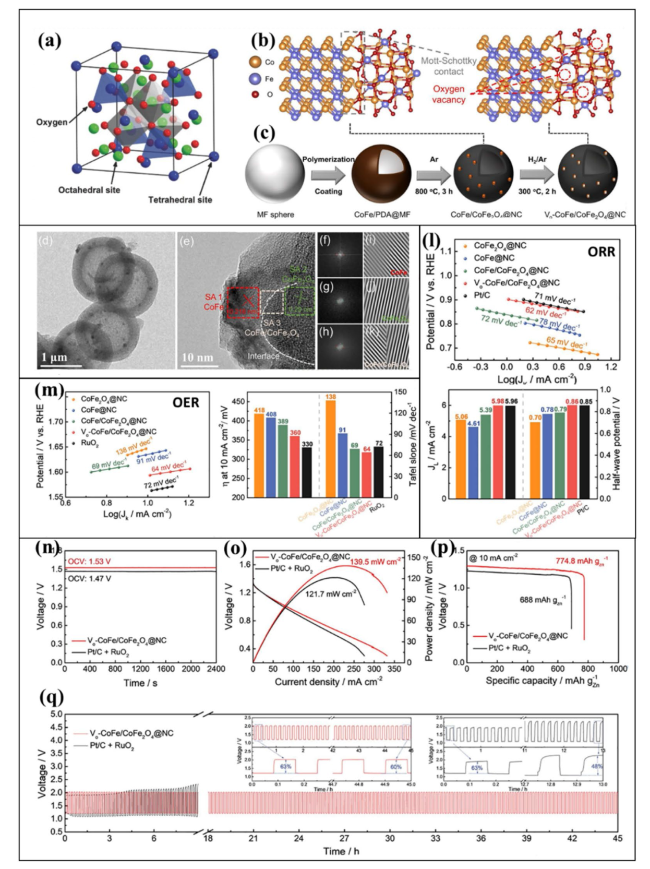
Fig. 7 a Illustration of the spinel crystal structure. Reproduced with permission from [154]. b, c Crystallographic arrangement and step-by-step illustration of the synthesis of hollow-structured Vo-CoFe/CoFe2O4@NC. d Low-resolution TEM image showing the hollow structure of Vo-CoFe/CoFe2O4@NC. e HRTEM micrograph indicating the presence of CoFe, CoFe2O4, and CoFe/CoFe2O4 and the location of the heterointerface between CoFe and CoFe2O4. f-h Corresponding fast Fourier transform (FFT) and i-k inversed FFT micrographs. l ORR performance in terms of the Tafel plot (top) and the current density and half-wave potential (bottom) for the prepared catalysts in 0.1 M KOH with O2 saturation. m OER performance in terms of the Tafel plot (left), the OER overpotential (right) required to achieve a current of 10 mA cm−2, and the Tafel slopes for the prepared catalysts in 0.1 M KOH with N2 saturation. n Comparative analysis of the open-circuit voltage (OCV) measured for Vo-CoFe/CoFe2O4@NC and Pt/C + RuO2. o Polarization curve and plots of the power density. p Galvanostatic full-discharge test at a fixed current density of 10 mA cm.−2. q Charge/discharge cyclic performance for ZABs using Vo-CoFe/CoFe2O4@NC (red line) and Pt/C + RuO2 (black line) as the air cathode. Reproduced with permission from [158] |
4.2 Perovskite Oxides Electrocatalysts in Alkaline Electrolytes
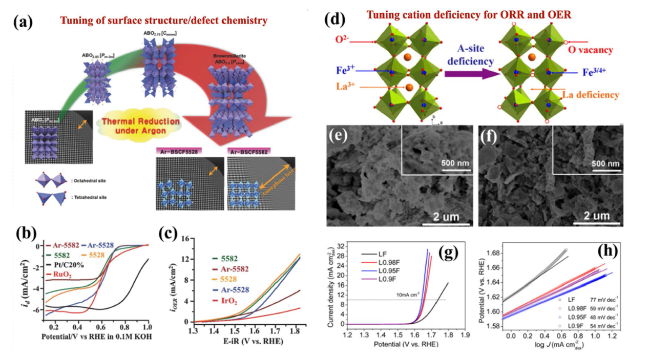
Fig. 8 a Structural change in response to heat treatment (950 °C/24 h) in an argon (Ar) environment. b ORR performance of modified perovskites and standard catalysts. c Linear plots for OER activity. Reproduced with permission from [156]. d A-site cationic deficiency strategy showing the crystal structures for the original LF and the oxygen vacancies in the modified La1-xFeO3-δ perovskites. e, f SEM micrographs for the original LF (left) and optimal perovskite L0.95F (right). g Linear voltammograms used to determine the OER performance of the original and modified perovskite catalysts and h corresponding Tafel plots. Reproduced with permission from [168] |
4.3 Carbon-Based Electrocatalysts for ZABs
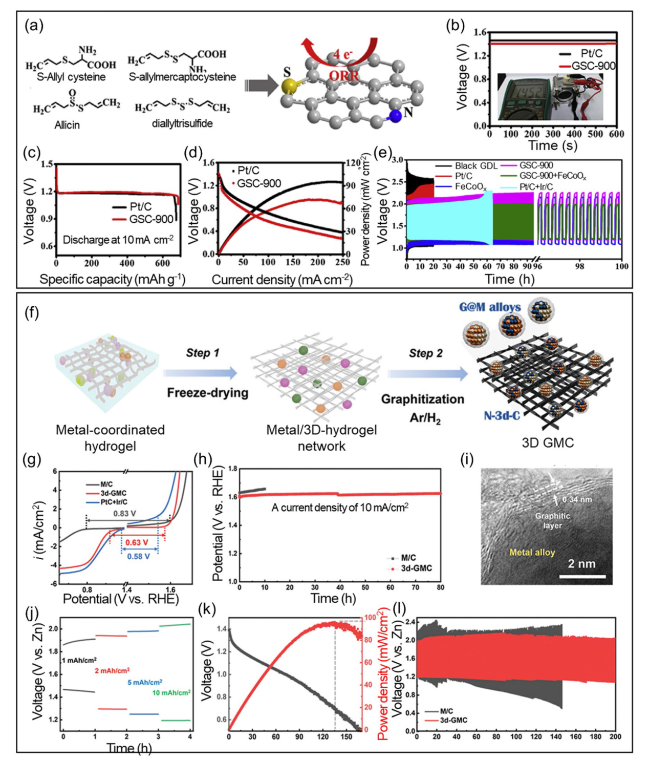
Fig. 9 a Preparation process for heteroatom (N and S) co-doped porous carbon derived from garlic stems. b OCV for a primary ZAB containing GSC-900 and Pt/C as air cathodes. The inset presents the voltage measured using a multimeter. c Galvanostatic discharge curves for the primary ZABs to assess their specific capacity. d V-J measurements and related power density for ZABs using GSC-900 and Pt/C as air cathodes. e Galvanostatic cycles for different electrocatalysts and their physical mixtures when used as the air cathode in a rechargeable ZAB. f Schematic illustration of the simple preparation process of 3d-GMC from the metal-coordinated hydrogel. g Bifunctionality of M/C (black), 3d-GMC (red), and Pt/C + Ir/C (blue): the bifunctionality value (i.e., onset potential differences (ΔE) between ORR and OER) for each catalyst is provided in figs. h Durability test of M/C (black) and 3d-GMC (red) at a current density of 10 mA cm−2. The electrocatalytic test was performed using 0.1 m KOH as an electrolyte. i TEM images for 3d-GMC after OER test. Full cell performance of 3d-GMC for Zn-air battery. j Rate capability, k polarization tests demonstrating power density, and l cyclability for a Zn-air battery assembled using a 3d-GMC cathode (red) and commercial Zn foil anode compared with the Zn-air battery using M/C as the cathode (black). The rate capability test was conducted in this order: OCV, 1, 2, 5, 10 mA cm.−2. Reproduced with permission from [179] |
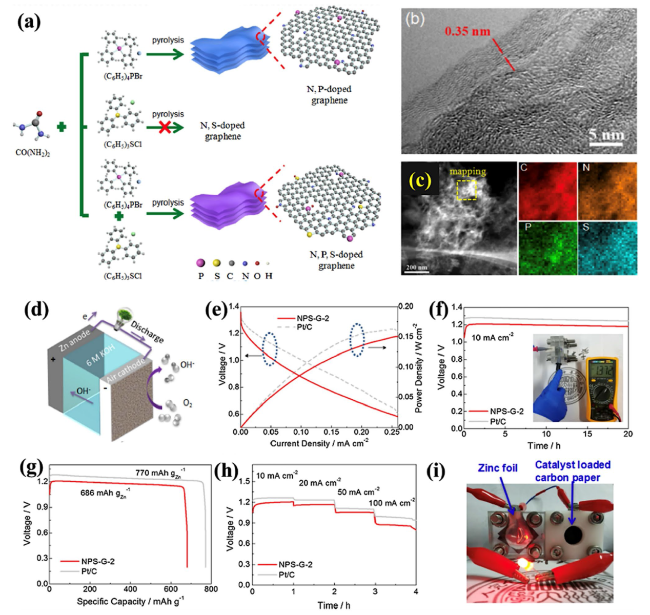
Fig. 10 a Process for the fabrication of N,P-doped graphene, N,S-doped graphene and N,S,P-doped graphene (NSP-G). b HRTEM micrograph of NSP-G. c STEM micrograph of the prepared NPS-G sample and elemental mapping to determine the heteroatom content (N, P, and S). d 3D diagram of the ZAB. e Power density calculations and polarization curves for ZABs using NPS-G-2 and commercial Pt/C (with 20 wt.%) as the air cathode. f Galvanostatic discharge curves at 10 mA cm.−2 for the ZABs with NPS-G-2 and Pt/C as the air cathodes, showing an OCV of 1.372 V for NPS-G-2. g Specific capacity of the ZABs with NPS-G-2 and Pt/C as the ORR catalyst. h Discharge profiles for different current densities for the ZABs with NPS-G-2 and Pt/C as the air catalyst. i Photographic image showing illumination from a green LED powered by two liquid ZABs connected in series with NPS-G-2 as the air cathode. Reproduced with permission from [185] |
4.4 Hybrid or Mixed Electrocatalysts for ZABs
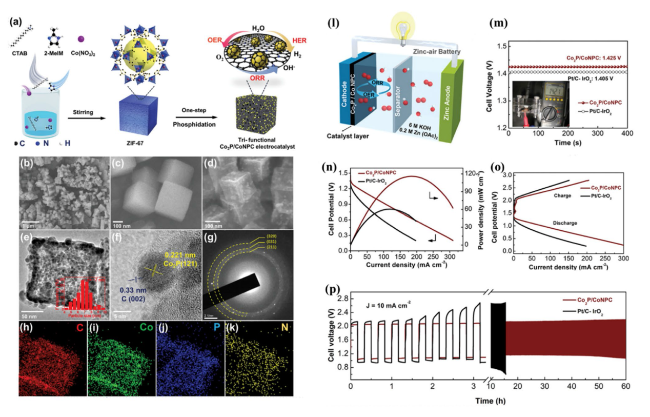
Fig. 11 a Preparation process for Co2P/CoNPC. b, c SEM images for ZIF-67. d SEM image for Co2P/CoNPC. e HRTEM image for Co2P/CoNPC, with the inset showing the particle size distribution for Co2P NPs. f HRTEM image showing the planes related to Co2P NPs and the carbon framework. g SAED pattern. h-k Elemental mapping of Co2P/CoNPC. l Co2P/CoNPC used as the air cathode in a ZAB. m OCV plot with the inset showing the multimeter setup for the calculation of the voltage. n Plot for the power density. o Charge/discharge polarization data. p Cyclic stability performance for the assembled ZAB. Reproduced with permission from [194] |
Table 3 Recent progress of bifunctional catalysts with their performance in ZABs |
| Type | Electrolyte | Air electrode catalyst layer | Zn electrode | Open circuit voltage (V) | Discharge/charge voltage gap (V) | Peak power density (mW cm−2) | Specific capacity (mAh g−1) | Cyclic performance | References |
|---|---|---|---|---|---|---|---|---|---|
| A | 6 M KOH + 0.2 M Zn(CH3COO)2∙2H2O | N-CoS2 YSSs | Polished Zn foil | 1.41 | 0.85 @10 mA cm−2 | 81 | 744 | More than 165 h @10 mA cm−2 | [202] |
| A | 6.0 M KOH + 0.2 M Zn acetate | Porous Ni/NiO nanosheets | Zn plate | 1.47 | 0.83 @2 mA cm−2 | 225 | 853 | 240 cycles, 120 h @2 mA cm−2 | [203] |
| A | poly(vinyl alcohol) (PVA) gel film | Spinel CoIn2Se4 nanosheets | Zn plate | 1.37 | 0.71 @10 mA cm−2 | 107 | 733 | 400 cycles @10 mA cm−2 | [204] |
| A | 6 M KOH + 0.2 M Zn acetate | MnO2-IL0.5 | Polished Zn plates | 1.51 | 0.86 @10 mA cm−2 | 166 | 762 | 40 h @10 mA cm−2 | [205] |
| A | KOH + Zn(Ac)2 | np-AlFeCoNiCr | Zn foil | 1.55 | 0.76 @2 mA cm−2 | 125 | 800 | 120 h @20 mA cm−2 | [206] |
| A,B | 6 M KOH + 0.2 M Zn(OAc)2 | Co3O4@LaCoO3 | Zn plate | 1.46 | - | 140 | 785 | 555 cycles, 185 h @2 mA cm−2 | [207] |
| A,B | 6 M KOH + 0.2 M Zn(OAc)2 | LaMnO3 | Zn foil | - | 0.82 @10 mA cm−2 | 170 | 725 | 100 cycles @5 mA cm−2 | [208] |
| A,B | 6 M KOH + 0.2 M Zn(OAc)2 | LaNi0.85Mg0.15O3 | Polished Zn foil | 1.35 | 0.92 @10 mA cm−2 | 45 | 810 | 220 cycles, 110 h @10 mA cm−2 | [209] |
| A,B | 6 M KOH + 0.2 M Zn(OAc)2 | Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF)-ceria (CeO2) | Zn foil | 1.62 | 0.83 @20 mA cm−2 | 131 | 716 | 180 cycles, 80 h @10 mA cm−2 | [210] |
| A,B,C,D | 6 M KOH + 0.2 M ZnCl2 | Pt-Sr(Co0.8Fe0.2)0.95P0.05O3−δ (SCFP)/Super P | Zn plate | 1.44 | 0.86 @5 mA cm−2 | 122 | 790.4 | 240 cycles, 80 h @5 mA cm−2 | [100] |
| A,B,C,D | 6 M KOH + 0.2 M Zn(OAc)2 | LaMn0.7Co0.3O3 (LMCO) | Zn foil | 1.40 | 0.77 @1 mA cm−2 | 35 | 764 | 90 cycles, 30 h @5 mA cm−2 | [211] |
| A,B,C,D | 6 M KOH + 0.2 M Zn(OAc)2 | Ni3FeN/V@N-doped graphene | Polished Zn foil | 1.52 | 0.92 @10 mA cm−2 | 168 | 650 | 150 cycles, 220 h @10 mA cm−2 | [212] |
| A,C,D | 6 M KOH | Fe@N-C-700 | Zn plate | 1.40 | - | 220 | - | 100 cycles, 16.7 h @10 mA cm−2 | [213] |
| A,C,D | 6 M KOH + 0.2 M Zn(CH3COO)2 | NCO@HHPC | Zn plate | 1.48 | 0.73 @10 mA cm−2 | 267 | 767 | 1460 cycles, 487 h @10 mA cm−2 | [214] |
| A,C,D | 6 M KOH + 0.2 M Zn acetate | Co5.47N@N-rGO-750 | Zn plate | 1.45 | 0.77 @1 mA cm−2 | 121 | 789 | 2000 cycles, 330 h @1 mA cm−2 | [215] |
| A,C,D | 6 M KOH + 0.2 M Zn(Ac)2 | FeNiP/NPCS | Polished Zn plate | 1.51 | 0.58 @10 mA cm−2 | 163 | 603 | 330 cycles, 110 h @10 mA cm−2 | [216] |
| A,C,D | 6 M KOH/0.2 M Zn(ac)2 mixed solution | Ni-Co-S/NSC | Zn plate | 1.43 | 0.73 @10 mA cm−2 | 137 | 829 | 180 cycles @10 mA cm−2 | [217] |
| A,C,D | 6 M KOH + 0.2 M Zn(CH3COO)2 | GNCNTs-4 | Polished Zn foil | 1.48 | 0.76 @5 mA cm−2 | 253 | 801 | 9000 cycles, 3000 h @5 mA cm−2 | [218] |
| A,C,D | 6.0 M KOH + 0.2 M Zn(Ac)2 | Ni1Co3@N-CN | Zn foil | 1.446 | 0.71 @5 mA cm−2 | 98.2 | 721.6 | 200 h @5 mA cm−2 | [219] |
| A,C,D | 6.0 M KOH + 0.2 M zinc acetate | NCNTM | Zn foil | 1.5 | 0.8 @5 mA cm−2 | 220 | 797 | 4800 cycles, 1600 h @5 mA cm−2 | [220] |
| A,C,D | 6 M KOH + 0.2 M Zn(Ac)2 | Co-Co3O4@NAC | Polished Zn plate | 1.45 | 0.77 @10 mA cm−2 | 164 | 721 | 35 h @10 mA cm−2 | [97] |
| A,C,D | 6 M KOH + 0.2 M ZnCl2 | Fe,Co-SA/CS | Polished Zn plate | 1.43 | 0.88 @5 mA cm−2 | 86.7 | 819.6 | 300 cycles, 100 h @5 mA cm−2 | [221] |
| A,C,D | 6 M KOH + 0.2 M ZnCl2 | Fe-Nx-HCS | Polished Zn plate | 1.42 | 1 @10 mA cm−2 | 154 | 422 | 58 h @10 mA cm−2 | [222] |
| A,C,D | 6.0 mol L−1 KOH + 0.2 mol L−1 Zn acetate | CoSe2@NC loaded on Ni foam | Polished Zn plates | 1.48 | 0.93 @10 mA cm−2 | 137.1 | 751.1 | 500 cycles @10 mA cm−2 | [223] |
| A,C,D | 6 M KOH + 0.2 M Zn (AC)2 | CoFe@NC/KB-800 | Zn foil | 1.351 | 0.65 @2 mA cm−2 | 160 | 654 | 600 cycles, 100 h @2 mA cm−2 | [224] |
| A,C,D | 0.2 M Zn(CH3COO)2 + 6 M KOH | MDPCF-based ZAB | Zn foil | 1.48 | - | 288.8 | 740 | 330 h @10 mA cm−2 | [225] |
| A,C,D | 6 M KOH | CoP/NP-HPC | Zn plate | 1.4 | - | 186 | - | 80 h @2 mA cm−2 | [226] |
| A,C,D | 6 M KOH + 0.2 M zinc acetate | RuCoOx@Co/N-CNT | Polished Zn plates | 1.44 | 0.79 @2 mA cm−2 | 93 | 788 | 200 cycles, 34 h @2 mA cm−2 | [227] |
| A,C,D | 6 M KOH + 0.2 M Zn acetate | P,S-CNS | Polished Zn plates | 1.51 | - | 198 | 830 | 200 cycles, 40 h @2 mA cm−2 | [228] |
| A,C,D | 6 M KOH + 0.2 M Zn(OAc)2 | MnCo2O4@C | Polished Zn plate | 1.43 | 0.72 @5 mA cm−2 | 40 | - | 70 h @10 mA cm−2 | [229] |
| A,C,D | 6 M KOH + 0.2 M Zn(CH3COO)2 | Co/Co3O4@PGS | Zn plate | 1.45 | 0.96 @20 mA cm−2 | 118.27 | - | 4800 cycles, 800 h @10 mA cm−2 | [136] |
| A,C,D | 6 M KOH + 0.2 M ZnCl2 | Fe0.5Co0.5Ox/NrGO | Zn plate | 1.43-1.44 | 0.89 @10 mA cm−2 | 86 | 756 | 120 h @10 mA cm−2 | [230] |
| A,C,D | 6 M KOH + 0.2 M zinc acetate | Janus NiFe@C@Co CNFs | Polished Zn foil | 1.44 | 0.77 @5 mA cm−2 | 130 | 694 | 200 h @5 mA cm−2 | [231] |
| A,C,D | 6 M KOH + 0.2 M ZnCl2 | FeCo@NC-d | Zn plate | 1.456 | 0.79 @5 mA cm−2 | 190.2 | - | 120 cycles @5 mA cm−2 | [232] |
| A,C,D | 6 M KOH + 0.2 M ZnO | CuCo2O4@CNTs | Zn plate | 1.41 | 0.79 @10 mA cm−2 | - | - | 160 cycles, 80 h @2 mA cm−2 | [233] |
| A,C,D | 6 M KOH + 0.2 M Zn(CH3COO)2 | Fe2Ni@NC | Zn plate | 1.493 | 0.805 @50 mA cm−2 | 126 | - | 500 cycles @10 mA cm−2 | [234] |
| A,C,D | KOH/Zn(Ac)2 | CoNi@CoCN | Zn plate | 1.498 | - | 162.5 | 773.5 | 200 cycles | [235] |
| A,C,D | 6 M KOH + 0.2 M Zn(CH3COO)2 | NPSC-Co2Fe1 | Zn foil | 1.44 | 0.96 @10 mA cm−2 | 174.6 | - | 210 cycles, 70 h @5 mA cm−2 | [236] |
| A,C,D | 6 M KOH + 0.2 M ZnCl2 | CoSx/Co-NC-800 | Zn foil | 1.40 | 0.73 @2 mA cm−2 | 103 | 770.4 | 450 cycles, 90 h @5 mA cm−2 | [237] |
| A,C,D | 6 M KOH + 0.2 M Zn(OAc)2 | FeCo-N-C-700 | Tailored Zn plate | 1.39 | - | 150 | 518 | 360 cycles, 60 h @1 mA cm−2 | [238] |
A: Bifunctional oxygen electrocatalyst, B: Perovskite oxides as electrocatalysts, C: Carbon-based electrocatalysts, D: Hybrid/mixed electrocatalysts |
5 Advanced Form of ZABs
5.1 Mechanically Rechargeable ZABs
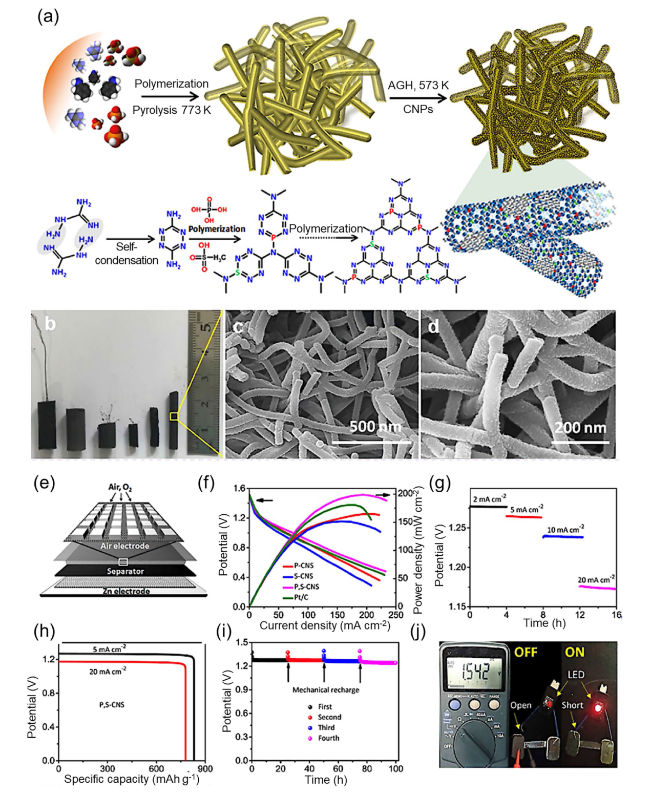
Fig. 12 a Synthesis process for the sponge-like P,S-CNS catalyst and associated reaction mechanisms. b Photograph of the prepared P,S-CNS samples and c, d corresponding SEM images. e Schematic diagram of the primary ZAB. f Polarization curves and calculation of the power densities for primary ZABs constructed using various catalysts. g Galvanostatic discharge curves for the primary ZAB using P,S-CNS as the air cathode. h Specific capacity of the primary ZAB using P,S-CNS as the ORR catalyst. i Stability of the primary ZAB using a P,S-CNS cathode with mechanical recharging. j Photograph of LED illumination powered by the proposed ZAB. Reproduced with permission from [228] |
5.2 Flexible Zn-Air Batteries
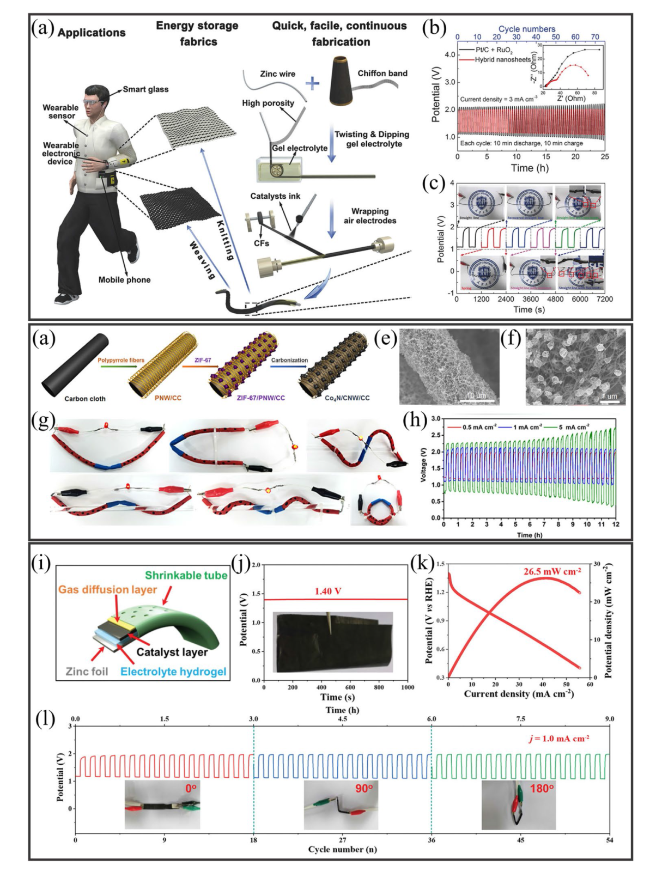
Fig. 13 a Fabrication process for the air electrode in a fiber-shaped flexible ZAB. b Galvanostatic charge-discharge plots for ZABs using different electrocatalysts. c Galvanostatic charge-discharge curves for a fiber-shaped flexible ZAB under different deformation conditions. Reproduced with permission from [254]. d Fabrication process for a Co4N/CNW/CC electrode. e, f Low- (10 µm) and high-resolution (1 µm) SEM images for the prepared Co4N/CNW/CC electrode. g Images of a cable-type flexible ZAB under different twisting or bending conditions. h Current density-dependent galvanostatic charge-discharge curves for the cable-type flexible ZAB. Reproduced with permission from [34]. i Schematic diagram of a flexible ZAB based on a Co/N@CNTs@CNMF-800 cathode. j Measurement of the OCV with the inset showing the flexible electrode. k Polarization and power density plots. l Cyclic stability of the flexible ZAB under different bending conditions. Reproduced with permission from [255] |
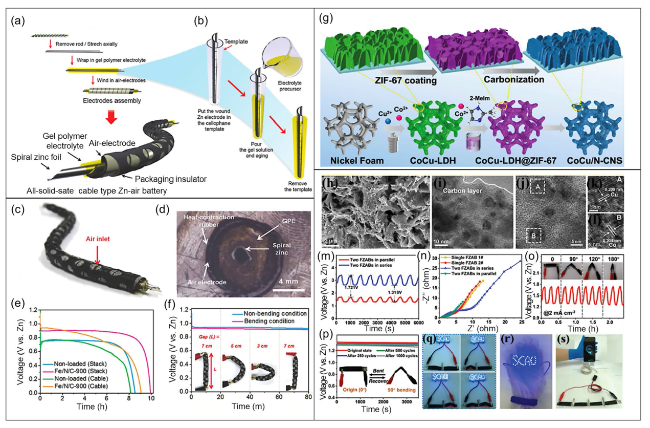
Fig. 14 a Fabrication process for a solid-state flexible cable-type ZAB. b Coating of gelatin-based GPE and KOH (0.1 M) on a spiral zinc anode. c Photograph of a prototype flexible cable-type ZAB. d Cross-sectional optical microscope image of the cable-type ZAB. e Discharge curves for cable-type and stacked ZABs with/without the Fe/N/C electrocatalyst measured at a current density of 0.1 mA cm−2. f Discharge curve measurements for a flexible cable-type ZAB under different bending conditions. Reproduced with permission from [36]. g Fabrication process for the synthesis of the electrocatalyst CoCu/N-CNS-x (x = 1, 2, 3) over nickel foam. h SEM image (2 µm). i TEM image (10 nm). j-l HRTEM images (5 nm and 1 nm) showing the presence of Cu and Co in the prepared electrocatalyst. m Measurement of charge-discharge plots for two flexible ZABs connected in parallel or series and n corresponding Nyquist plots. o Charge-discharge measurements for the flexible ZAB under different bending conditions at 2 mA cm.−2. p Voltage measurements for the flexible ZAB after bending and recovery. q Illumination of an LED powered by two flexible ZABs connected in series under various bending settings. r Image of a wearable bracelet containing a flexible solid-state ZAB used to power the LED screen. s Charging of a mobile phone using four ZABs in series. Reproduced with permission from [256] |