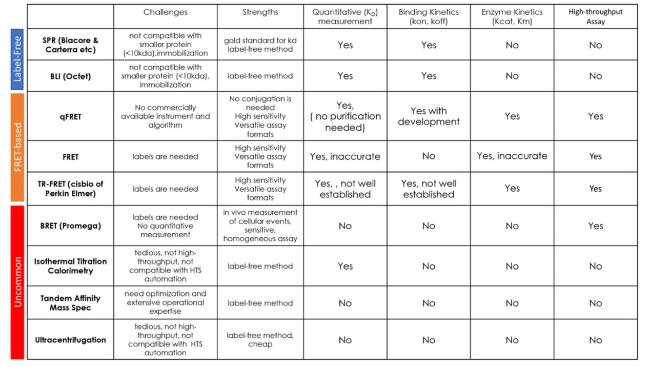
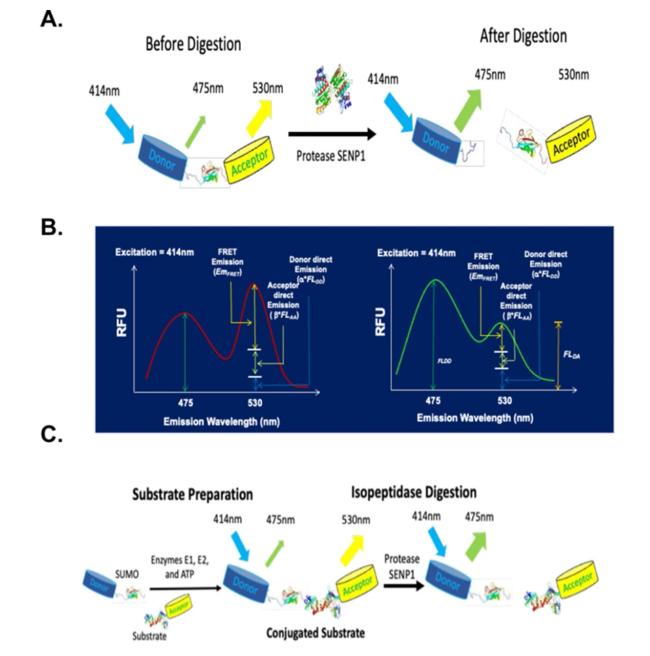
In addition to SPR, the ITC is also often used for
KD determination [
40,
52]. These methods offer experimental convenience, but also have some disadvantages. ITC directly measures heat release that are caused by the endothermic or exothermic reaction between two interacting molecules [
53,
54]. The ITC often requires environmentally unfriendly labeling and cannot determine impure protein interactions. ITC requires relatively large amounts (i.e., millimolar range) of highly purified proteins, which often are hard to obtain. Systematic errors in heart calibration, cell volume, and other errors (e.g., baseline and gas bubbles), can lead to inaccuracies in
KD values [
55⇓-
57]. In addition, ITC requires expensive specialized instrumentation. Other methods, such as radioactive labeling, ultracentrifugation, and fluorescence polarization, are also used to determine molecular interaction affinity with some limitations. For the ultracentrifugation assay, the elongated centrifugation in a non-physiological buffer can shift the equilibrium between bound and free proteins, especially for those proteins with fast dissociation rates; thus, the determined
KD values may not represent true equilibrium constants. In addition, large amounts of proteins, which can be nonspecifically adsorbed on the walls of centrifuge tubes during high-speed centrifugation, are required for ultracentrifugation. In principle, the FP assay, which measures the changed of polarized light from one interactive partner after binding, can potentially address the above issues [
58]. The FP assay has successfully determined peptide or nucleic acid interaction dissociation constants and an HTS platform for small molecule drug screening. However, the FP method determines polarized light changes of free-rotated fluorescence molecules; therefore, one interactive partner must be small and attached to a fluorophore, significantly affecting the interaction affinity. When measuring large proteins, the FP assay becomes less sensitive [
59]. It also typically requires sensitive instruments for accurate quantification, such as fluorescence microscopy, preventing its high-throughput application.