

诊断学理论与实践 ›› 2022, Vol. 21 ›› Issue (06): 669-676.doi: 10.16150/j.1671-2870.2022.06.002
收稿日期:2022-10-03
出版日期:2022-12-25
发布日期:2023-04-23
通讯作者:
张超
E-mail:chaozhang@tmu.edu.cn
基金资助:Received:2022-10-03
Online:2022-12-25
Published:2023-04-23
Contact:
ZHANG Chao
E-mail:chaozhang@tmu.edu.cn
摘要:
多发性硬化(multiple sclerosis,MS)是一种自身免疫性慢性炎症性中枢神经系统脱髓鞘疾病。约95%的MS患者发病时为复发缓解型(relapsing remitting MS,RRMS),而其中多数RRMS患者会发展为继发进展型多发性硬化(secondary progressive MS,SPMS)。SPMS的发病机制目前尚不完全明确,但研究表明,SPMS的发生与中枢神经系统内缓慢扩张的病变、软脑膜滤泡样结构等机制相关,涉及驻留在中枢神经系统、软脑膜和脑脊液中的免疫细胞,造成神经元持续性变性损害和修复能力下降。在临床上,SPMS主要表现为患者残疾进展,但其诊断通常是回顾性的,仍然具有挑战性,因多达三分之二的具有潜在残疾恶化的患者仍被诊断为RRMS。此类患者继续接受针对RRMS的疾病修饰疗法可能效果欠佳。研究SPMS发病机制的有助于早期识别和诊断。
中图分类号:
张超, 高雪. 继发进展型多发性硬化的临床诊断进展[J]. 诊断学理论与实践, 2022, 21(06): 669-676.
ZHANG Chao, GAO Xue. Advances in clinical diagnosis of secondary progressive multiple sclerosis[J]. Journal of Diagnostics Concepts & Practice, 2022, 21(06): 669-676.
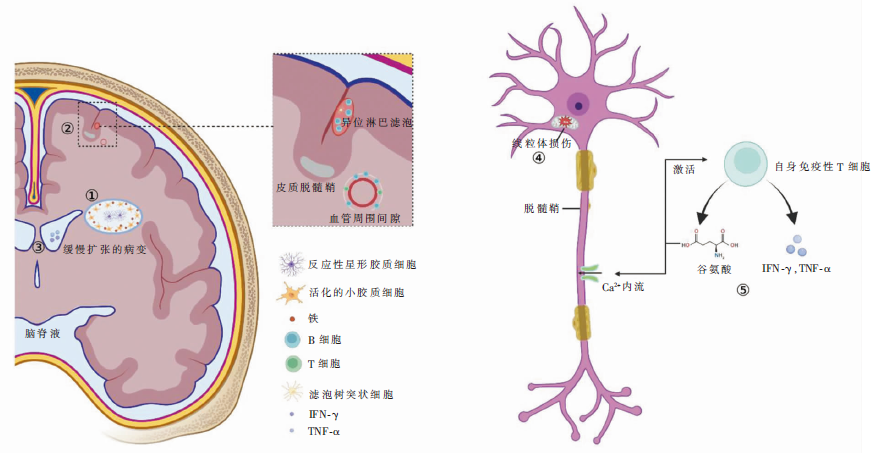
图1
SPMS的简要发病机制注:①缓慢扩张的病变边缘积聚活化的小胶质细胞,同时,铁在病变边缘活化的小胶质细胞中高度富集;位于缓慢扩张病变的非活性病变中心的反应性星形胶质细胞在脱髓鞘的轴突周围形成胶质瘢痕组织。②异位淋巴滤泡与严重的皮质脱髓鞘有关,主要位于脑膜进入脑沟处的蛛网膜下腔邻近的软脑膜下皮质,为大型B细胞聚集体(主要包含记忆B细胞),并存在表达CXCL13的基质及滤泡树突状细胞;同时,CD20+ B细胞也聚集在白质的血管周围间隙,驱动白质脱髓鞘。③脑脊液中含有的与疾病进展和梯度损伤相关的可溶性分子,包括IFN-γ和TNF-α,在进展型MS患者脑表面皮质和脑室周围损伤中具有关键作用。④线粒体对氧化损伤高度敏感,活性氧和活性氮会损害线粒体呼吸链复合体的活性,导致能量衰竭,从而使轴突变性和神经细胞死亡。⑤自身免疫性T细胞可产生谷氨酸盐,谷氨酸受体的持续激活会导致轴突内钙离子过量、离子失衡,导致轴突变性;谷氨酸盐也会激活自身免疫性T细胞分泌促炎细胞因子,如TNF-α和IFN-γ,从而增加神经炎症。
| [1] |
Inojosa H, Proschmann U, Akgün K, et al. A focus on secondary progressive multiple sclerosis (SPMS): challenges in diagnosis and definition[J]. J Neurol, 2021, 268(4):1210-1221.
doi: 10.1007/s00415-019-09489-5 |
| [2] |
Weinshenker B G, Bass B, Rice G P, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability[J]. Brain, 1989, 112 (Pt 1):133-146.
doi: 10.1093/brain/112.1.133 URL |
| [3] |
Healy L M, Stratton J A, Kuhlmann T, et al. The role of glial cells in multiple sclerosis disease progression[J]. Nat Rev Neurol, 2022, 18(4):237-248.
doi: 10.1038/s41582-022-00624-x pmid: 35190704 |
| [4] |
Yong H Y F, Yong V W. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis[J]. Nat Rev Neurol, 2022, 18(1):40-55.
doi: 10.1038/s41582-021-00581-x |
| [5] |
Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis[J]. Nat Rev Neurol, 2012, 8(11):647-656.
doi: 10.1038/nrneurol.2012.168 pmid: 23007702 |
| [6] |
Correale J, Gaitán M I, Ysrraelit M C, et al. Progressive multiple sclerosis: from pathogenic mechanisms to treatment[J]. Brain, 2017, 140(3):527-546.
doi: 10.1093/brain/aww258 pmid: 27794524 |
| [7] |
Oki S. Eomes-expressing T-helper cells as potential target of therapy in chronic neuroinflammation[J]. Neurochem Int, 2019, 130:104348.
doi: 10.1016/j.neuint.2018.11.023 URL |
| [8] |
Peruzzotti-Jametti L, Willis C M, Hamel R, et al. Metabolic Control of Smoldering Neuroinflammation[J]. Front Immunol, 2021, 12:705920.
doi: 10.3389/fimmu.2021.705920 URL |
| [9] |
Jäckle K, Zeis T, Schaeren-Wiemers N, et al. Molecular signature of slowly expanding lesions in progressive multiple sclerosis[J]. Brain, 2020, 143(7):2073-2088.
doi: 10.1093/brain/awaa158 pmid: 32577755 |
| [10] |
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging[J]. Acta Neuropathol, 2017, 133(1):25-42.
doi: 10.1007/s00401-016-1636-z pmid: 27796537 |
| [11] |
Gillen K M, Mubarak M, Nguyen T D, et al. Significance and In Vivo Detection of Iron-Laden Microglia in White Matter Multiple Sclerosis Lesions[J]. Front Immunol, 2018, 9:255.
doi: 10.3389/fimmu.2018.00255 pmid: 29515576 |
| [12] |
Mehta V, Pei W, Yang G, et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions[J]. PLoS One, 2013, 8(3):e57573.
doi: 10.1371/journal.pone.0057573 URL |
| [13] |
Popescu B F, Frischer J M, Webb S M, et al. Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions[J]. Acta Neuropathol, 2017, 134(1):45-64.
doi: 10.1007/s00401-017-1696-8 pmid: 28332093 |
| [14] |
Lassmann H. Targets of therapy in progressive MS[J]. Mult Scler, 2017, 23(12):1593-1599.
doi: 10.1177/1352458517729455 URL |
| [15] |
Choi I Y, Lee P, Adany P, et al. In vivo evidence of oxi-dative stress in brains of patients with progressive multiple sclerosis[J]. Mult Scler, 2018, 24(8):1029-1038.
doi: 10.1177/1352458517711568 URL |
| [16] |
Rawji K S, Gonzalez Martinez G A, Sharma A, et al. The Role of Astrocytes in Remyelination[J]. Trends Neurosci, 2020, 43(8):596-607.
doi: S0166-2236(20)30124-7 pmid: 32620289 |
| [17] |
Faissner S, Plemel J R, Gold R, et al. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies[J]. Nat Rev Drug Discov, 2019, 18(12):905-922.
doi: 10.1038/s41573-019-0035-2 pmid: 31399729 |
| [18] |
Reali C, Magliozzi R, Roncaroli F, et al. B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis[J]. Brain Pathol, 2020, 30(4):779-793.
doi: 10.1111/bpa.v30.4 URL |
| [19] |
Lassmann H. Pathogenic Mechanisms Associated With Different Clinical Courses of Multiple Sclerosis[J]. Front Immunol, 2019, 9:3116.
doi: 10.3389/fimmu.2018.03116 URL |
| [20] |
Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology[J]. Brain, 2007, 130(Pt 4):1089-1104.
doi: 10.1093/brain/awm038 pmid: 17438020 |
| [21] |
Serafini B, Rosicarelli B, Magliozzi R, et al. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis[J]. Brain Pathol, 2004, 14(2):164-174.
doi: 10.1111/j.1750-3639.2004.tb00049.x pmid: 15193029 |
| [22] |
Aloisi F, Serafini B, Magliozzi R, et al. Detection of Epstein-Barr virus and B-cell follicles in the multiple sclerosis brain: what you find depends on how and where you look[J]. Brain, 2010, 133(Pt 12):e157.
doi: 10.1093/brain/awq223 URL |
| [23] |
Moccia M, Haider L, Eshaghi A, et al. B Cells in the CNS at Postmortem Are Associated With Worse Outcome and Cell Types in Multiple Sclerosis[J]. Neurol Neuroimmunol Neuroinflamm, 2021, 9(1):e1108.
doi: 10.1212/NXI.0000000000001108 URL |
| [24] |
Gardner C, Magliozzi R, Durrenberger P F, et al. Cortical grey matter demyelination can be induced by elevated pro-inflammatory cytokines in the subarachnoid space of MOG-immunized rats[J]. Brain, 2013, 136(Pt 12):3596-3608.
doi: 10.1093/brain/awt279 pmid: 24176976 |
| [25] | Raveney B J E, Sato W, Takewaki D, et al. Involvement of cytotoxic Eomes-expressing CD4+ T cells in secondary progressive multiple sclerosis[J]. Proc Natl Acad Sci U S A, 2021, 118(11):e2021818118. |
| [26] |
Raveney B J, Oki S, Hohjoh H, et al. Eomesodermin-expressing T-helper cells are essential for chronic neuroinflammation[J]. Nat Commun, 2015, 6:8437.
doi: 10.1038/ncomms9437 pmid: 26436530 |
| [27] | Levite M. Glutamate, T cells and multiple sclerosis[J]. J Neural Transm (Vienna), 2017, 124(7):775-798. |
| [28] |
Scalfari A, Neuhaus A, Daumer M, et al. Onset of secondary progressive phase and long-term evolution of multiple sclerosis[J]. J Neurol Neurosurg Psychiatry, 2014, 85(1):67-75.
doi: 10.1136/jnnp-2012-304333 pmid: 23486991 |
| [29] | Ciron J, Gueguen A, Al K A, et al. Secondary progressive multiple sclerosis: A national consensus paper on diagnostic criteria[J]. Rev Neurol (Paris), 2022. |
| [30] |
Katz S I, Krieger S, Farrell C, et al. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis[J]. Mult Scler, 2014, 20(12):1654-1657.
doi: 10.1177/1352458514521517 URL |
| [31] |
Rojas J I, Patrucco L, Alonso R, et al. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis: Multicenter study in Argentina[J]. Mult Scler, 2021, 27(4):579-584.
doi: 10.1177/1352458520924586 URL |
| [32] |
Cree B, Hollenbach J A, Bove R, et al. Silent progression in disease activity-free relapsing multiple sclerosis[J]. Ann Neurol, 2019, 85(5):653-666.
doi: 10.1002/ana.25463 pmid: 30851128 |
| [33] |
Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis[J]. Brain, 2016, 139(Pt 9):2395-2405.
doi: 10.1093/brain/aww173 pmid: 27401521 |
| [34] |
Filippi M, Preziosa P, Langdon D, et al. Identifying Progression in Multiple Sclerosis: New Perspectives[J]. Ann Neurol, 2020, 88(3):438-452.
doi: 10.1002/ana.v88.3 URL |
| [35] |
Magliozzi R, Fadda G, Brown R A, et al. "Ependymal-in" Gradient of Thalamic Damage in Progressive Multiple Sclerosis[J]. Ann Neurol, 2022, 92(4):670-685.
doi: 10.1002/ana.26448 pmid: 35748636 |
| [36] |
Kapoor R, Smith K E, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis[J]. Neurology, 2020, 95(10):436-444.
doi: 10.1212/WNL.0000000000010346 pmid: 32675076 |
| [37] |
Högel H, Rissanen E, Barro C, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity[J]. Mult Scler, 2020, 26(2):210-219.
doi: 10.1177/1352458518819380 URL |
| [38] |
Filippi M, Preziosa P, Barkhof F, et al. Diagnosis of Progressive Multiple Sclerosis From the Imaging Perspective: A Review[J]. JAMA Neurol, 2021, 78(3):351-364.
doi: 10.1001/jamaneurol.2020.4689 pmid: 33315071 |
| [39] | Klineova S, Lublin F D. Clinical Course of Multiple Sclerosis[J]. Cold Spring Harb Perspect Med, 2018, 8(9). |
| [40] | Preziosa P, Pagani E, Meani A, et al. Slowly Expanding Lesions Predict 9-Year Multiple Sclerosis Disease Progression[J]. Neurol Neuroimmunol Neuroinflamm, 2022, 9(2). |
| [41] |
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI[J]. Brain, 2021, 144(3):833-847.
doi: 10.1093/brain/awaa436 pmid: 33484118 |
| [42] |
Ontaneda D. Progressive Multiple Sclerosis[J]. Continuum (Minneap Minn), 2019, 25(3):736-752.
doi: 10.1212/CON.0000000000000727 pmid: 31162314 |
| [43] |
Genovese A V, Hagemeier J, Bergsland N, et al. Atrophied Brain T2 Lesion Volume at MRI Is Associated with Disability Progression and Conversion to Secondary Progressive Multiple Sclerosis[J]. Radiology, 2019, 293(2):424-433.
doi: 10.1148/radiol.2019190306 pmid: 31549947 |
| [44] |
Sucksdorff M, Matilainen M, Tuisku J, et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis[J]. Brain, 2020, 143(11):3318-3330.
doi: 10.1093/brain/awaa275 pmid: 33006604 |
| [45] |
Cree B, Arnold D L, Chataway J, et al. Secondary Progressive Multiple Sclerosis: New Insights[J]. Neurology, 2021, 97(8):378-388.
doi: 10.1212/WNL.0000000000012323 pmid: 34088878 |
| [46] | Nylund M, Sucksdorff M, Matilainen M, et al. Phenotyping of multiple sclerosis lesions according to innate immune cell activation using 18 kDa translocator protein-PET[J]. Brain Commun, 2022, 4(1):b301. |
| [47] |
Varhaug K N, Torkildsen Ø, Myhr K M, et al. Neurofilament Light Chain as a Biomarker in Multiple Sclerosis[J]. Front Neurol, 2019, 10:338.
doi: 10.3389/fneur.2019.00338 pmid: 31024432 |
| [48] |
Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa[J]. Clin Chem Lab Med, 2016, 54(10):1655-1661.
doi: 10.1515/cclm-2015-1195 pmid: 27071153 |
| [49] | Comabella M, Sastre-Garriga J, Carbonell-Mirabent P, et al. Serum neurofilament light chain levels predict long-term disability progression in patients with progressive multiple sclerosis[J]. J Neurol Neurosurg Psychiatry, 2022. |
| [50] |
Edwards K R, Kamath A, Button J, et al. A pharmacokinetic and biomarker study of delayed-release dimethyl fumarate in subjects with secondary progressive multiple sclerosis: evaluation of cerebrospinal fluid penetration and the effects on exploratory biomarkers[J]. Mult Scler Relat Disord, 2021, 51:102861.
doi: 10.1016/j.msard.2021.102861 URL |
| [51] |
Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis[J]. Brain, 2018, 141(8):2382-2391.
doi: 10.1093/brain/awy154 pmid: 29860296 |
| [52] |
Salzer J, Svenningsson A, Sundström P. Neurofilament light as a prognostic marker in multiple sclerosis[J]. Mult Scler, 2010, 16(3):287-292.
doi: 10.1177/1352458509359725 URL |
| [53] |
Sun M, Liu N, Xie Q, et al. A candidate biomarker of glial fibrillary acidic protein in CSF and blood in differentiating multiple sclerosis and its subtypes: A systematic review and meta-analysis[J]. Mult Scler Relat Disord, 2021, 51:102870.
doi: 10.1016/j.msard.2021.102870 URL |
| [54] |
Groen K, Lechner-Scott J, Pohl D, et al. Can serum glial fibrillary acidic protein(GFAP) solve the longstanding problem of diagnosis and monitoring progressive multiple sclerosis[J]. Mult Scler Relat Disord, 2021, 50:102931.
doi: 10.1016/j.msard.2021.102931 URL |
| [55] |
Saraste M, Bezukladova S, Matilainen M, et al. Increased serum glial fibrillary acidic protein associates with microstructural white matter damage in multiple sclerosis: GFAP and DTI[J]. Mult Scler Relat Disord, 2021, 50:102810.
doi: 10.1016/j.msard.2021.102810 URL |
| [56] |
Choi I Y, Lee P, Hughes A J, et al. Longitudinal changes of cerebral glutathione(GSH) levels associated with the clinical course of disease progression in patients with secondary progressive multiple sclerosis[J]. Mult Scler, 2017, 23(7):956-962.
doi: 10.1177/1352458516669441 URL |
| [57] |
Cristofanilli M, Gratch D, Pagano B, et al. Transglutaminase-6 is an autoantigen in progressive multiple sclerosis and is upregulated in reactive astrocytes[J]. Mult Scler, 2017, 23(13):1707-1715.
doi: 10.1177/1352458516684022 URL |
| [1] | 何亲羽, 王伟, 陈立芬, 张雪蕾, 董治亚. LHCGR基因突变致家族性男性性早熟2例报告及文献复习[J]. 诊断学理论与实践, 2022, 21(05): 598-605. |
| [2] | 陈志敏, 何浩岚. 艾滋病合并马尔尼菲篮状菌病的诊治现状[J]. 诊断学理论与实践, 2022, 21(04): 425-430. |
| [3] | 沈银忠. 《人类免疫缺陷病毒感染/艾滋病合并结核分枝杆菌感染诊治专家共识》解读[J]. 诊断学理论与实践, 2022, 21(04): 431-436. |
| [4] | 陈宏, 沈银忠. 人类免疫缺陷病毒感染/艾滋病合并结核病的诊治进展[J]. 诊断学理论与实践, 2022, 21(04): 530-534. |
| [5] | 何新, 陈慧, 冯炜炜. 机器学习算法在辅助超声诊断附件肿块良恶性中的应用研究进展[J]. 诊断学理论与实践, 2022, 21(04): 541-546. |
| [6] | 徐子真, 李擎天, 刘湘帆, 李莉, 李惠, 王也飞, 吴洁敏, 陈宁, 梁璆荔, 陈松立, 戴健敏, 宋珍, 丁磊. 实验诊断学在线课程的建立和实践[J]. 诊断学理论与实践, 2022, 21(04): 547-550. |
| [7] | 赵然, 詹维伟, 侯怡卿. 计算机辅助诊断系统辅助超声诊断甲状腺弥漫性病变合并结节良恶性的应用价值[J]. 诊断学理论与实践, 2022, 21(03): 390-394. |
| [8] | 郭业兵, 郑金峰. 阴道壁胃肠道外间质瘤一例报道并文献复习[J]. 诊断学理论与实践, 2022, 21(03): 405-407. |
| [9] | 王刚, 陈生弟. 神经病学的诊断:起源、发展及挑战[J]. 诊断学理论与实践, 2022, 21(01): 1-4. |
| [10] | 唐静仪, 余群, 刘军. 结合人工智能的结构影像分析对阿尔茨海默病的早期预测及精准诊断研究进展[J]. 诊断学理论与实践, 2022, 21(01): 12-17. |
| [11] | 魏文石. 直面我国阿尔茨海默病诊治的挑战——《中国阿尔茨海默病报告2021》解读[J]. 诊断学理论与实践, 2022, 21(01): 5-7. |
| [12] | 王蔚, 王小钦. 缺铁性贫血的病因诊断[J]. 诊断学理论与实践, 2021, 20(06): 529-532. |
| [13] | 岳婧婧, 宋琦, 江旭峰, 王黎, 赵维莅, 严福华. 磁共振全身扩散加权成像结合T2WI抑脂序列与FDG-PET/CT在初发淋巴瘤分期及病灶检出的对比研究[J]. 诊断学理论与实践, 2021, 20(06): 540-546. |
| [14] | 王昭晖, 吴海波. 胃神经鞘瘤31例临床病理学分析[J]. 诊断学理论与实践, 2021, 20(06): 552-556. |
| [15] | 王广宇, 杨昕, 张立娟, 谭姣容. 住院新诊断2型糖尿病男性患者血浆总睾酮水平与骨钙素的相关性研究[J]. 诊断学理论与实践, 2021, 20(06): 573-578. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
