

Journal of Diagnostics Concepts & Practice ›› 2025, Vol. 24 ›› Issue (04): 383-392.doi: 10.16150/j.1671-2870.2025.04.004
• Expert forum • Previous Articles Next Articles
ZHANG Pingxin1,2, YANG Jie3a, WANG Yangdi3b, CHEN Minhu2,3a, LI Xuehua3b, MAO Ren3a( )
)
Received:2025-05-22
Revised:2025-07-10
Accepted:2025-08-05
Online:2025-08-25
Published:2025-09-09
Contact:
MAO Ren
E-mail:maor5@mail.sysu.edu.cn
CLC Number:
ZHANG Pingxin, YANG Jie, WANG Yangdi, CHEN Minhu, LI Xuehua, MAO Ren. Research progress on noninvasive quantitative diagnosis of intestinal fibrosis in Crohn's disease[J]. Journal of Diagnostics Concepts & Practice, 2025, 24(04): 383-392.
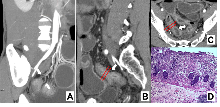
Figure 1
Intestinal fibrosis in CD characterized by MCFI Note: CTE image of a 33 year old male CD patient. Three dimensional reconstruction of blood vessels using MIP (maximum density projection) (A), sagittal (B), and transverse (C) enhanced CT images showed thickening of the intestinal wall and narrowing of the ileal lumen (indicated by arrows); The MCFI score of 2 was obtained from the designated segment reconstructed from adjacent mesenteric vessels, and the diagnosis was mild fibrosis, consistent with the histopathological (D) results.
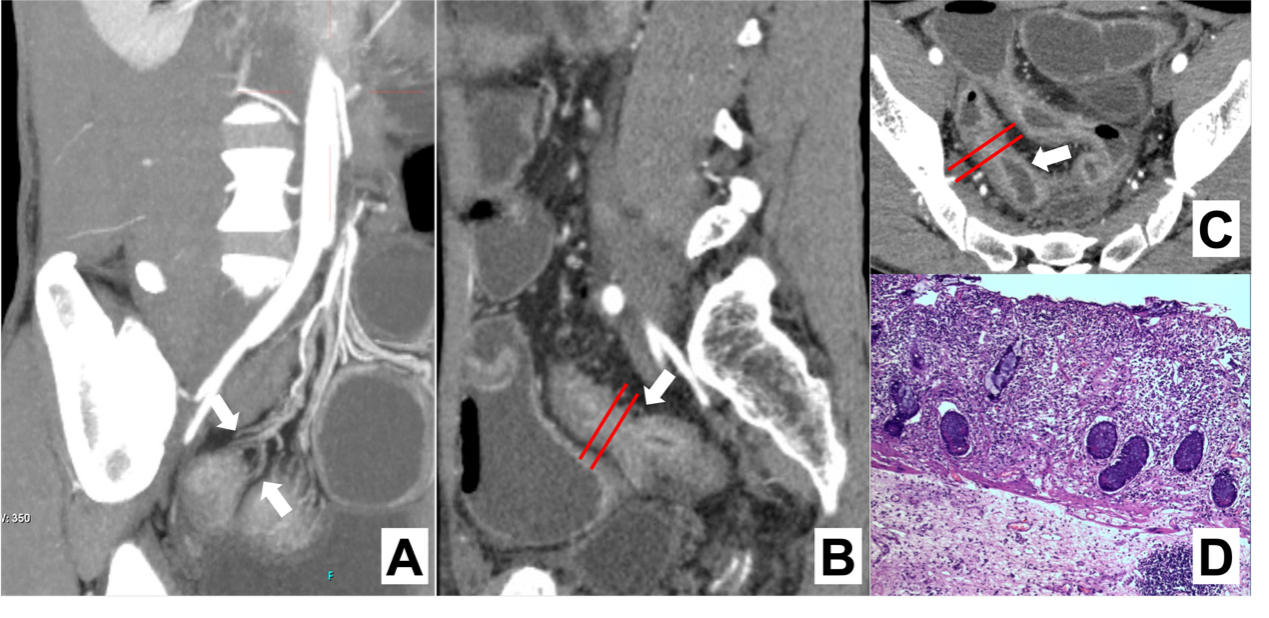
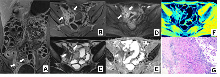
Figure 2
Intestinal fibrosis in CD characterized by MRE-based feature sequences Note: MRE image of a 29 year old male CD patient. Coronary T1WI enhanced scan (A), transverse T1WI enhanced scan (B), and transverse T2WI (C) indicate significant thickening of the intestinal wall at the lesion site; DWI value (D) increases; The ADC value (E) decreased to 0.995; MTI (F) indicates that the magnetization transfer rate of the intestinal wall at this location is MTR=41.16, the same level muscle MTR=50.02, and the normalized MTR (intestinal wall MTR at the lesion site/same level muscle MTR) is 0.82; The diagnosis of this case is moderate to severe fibrosis, consistent with histopathological (G) results.
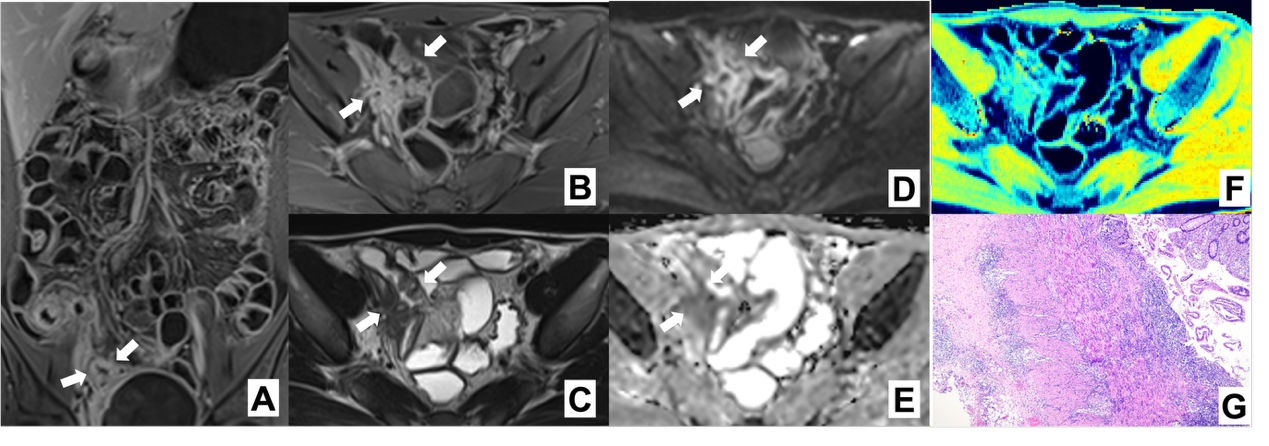
Table 1
Comparison of various cross-sectional imaging modalities for assessing intestinal fibrosis in CD
| 检查前准备复杂程度* | 检查耗时 | 检查成本* | 实施难点 | 适用场景 | |
|---|---|---|---|---|---|
| CTE | 4 | 1~2 h | 3 | ·设备可及性 ·患者配合度和依从性 | ·评估肠道全局病变 ·术前精准评估纤维化程度 |
| MRE | 4 | 2~3 h | 4 | ·设备可及性 ·患者配合度和依从性 | ·评估肠道全局病变 ·术前精准评估纤维化程度 ·有CTE检查禁忌 |
| IUS | 1 | 15~30 min | 2 | ·检查实施者技术经验 | ·狭窄患者初步筛查 ·门诊检查 ·病情随访 |
| PET-CT / PET-MRE | 4 | 1~3 h | 5 | ·设备可及性 ·患者配合度和依从性 ·检查实施者技术水平 ·检查成本 | ·术前精准分型 ·新药疗效评估 ·临床科研 |
Table 2
Summary of quantitative imaging metrics and predictive models for assessing intestinal fibrosis in CD
| 作者(年份) | 分类体系 | 样本量 | 预测参数 | 参考标准 | 纤维化分级 | 效能评估 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | 灵敏度 | 特异度 | |||||||||||||||
| CTE | |||||||||||||||||
| Huang, et al. (2025)[ | 肠道纤维化诊断模型 | 训练集:6 验证集:6 | ·电子密度 ·HU值 | 组织病理学 | 模型概率 ≤0.484,无/轻度纤维化 模型概率 >0.484,中/重度纤维化 | 0.828 | 77.3% | 82.4% | |||||||||
| Li, et al. (2021)[ | 放射组学模型 | 训练集:98 验证集:114 | 影像学特征值 | 组织病理学 | 模型概率 ≤0.811,轻度纤维化 模型概率 >0.811,中/重度纤维化 | 0.888 | 81.5% | 93.9% | |||||||||
| Meng, et al. (2022)[ | 深度学习模型 | 训练集:159 验证集:153 | ·肠壁厚度 ·壁层分层 ·狭窄前扩张 ·管腔大小 | 组织病理学 | 模型概率 ≤0.623,轻度纤维化 模型概率 >0.623,中/重度纤维化 | 0.828 | 78.0% | 85.7% | |||||||||
| Li, et al. (2021)[ | 肠系膜爬行脂肪指数 | 训练集:91 验证集:30 | 肠系膜爬行脂肪指数(MFCI) | 组织病理学 | MCFI ≤ 3分,轻度纤维化 MCFI >3分,中/重度纤维化 | 0.799 | 92.3% | 58.8% | |||||||||
| Meng, et al. (2022)[ | 列线图 | 训练集:91 验证集:83 | ·MFCI ·肠系膜水肿程度 ·病程 | 组织病理学 | 无/轻度纤维化 中/重度纤维化 | 0.832 | - | - | |||||||||
| MRE | |||||||||||||||||
| Coimbra, et al.(2022)[ | 综合诊断模型 | 61 | ·表观扩散系数 ·磁共振活动指数 ·延迟强化增益 | 组织病理学 | MRE纤维化评分 ≤2.1,轻度纤维化 MRE纤维化评分 >2.1,中/重度纤维化 | 0.910 | 68.1% | 100% | |||||||||
| Du, et al. (2021)[ | 表观扩散丰度成像 | 训练集:31 验证集:9 | 表观扩散峰度 | 组织病理学 | Kapp <0.775,无/轻度纤维化 Kapp ≥0.775,中/重度纤维化 | 0.896 | 95.9% | 78.1% | |||||||||
| Du, et al. (2020)[ | 表观扩散丰度成像 | 训练集:31 验证集:9 | ·表观扩散峰度 ·非高斯分布的表观扩散系数 | 组织病理学 | 总分对应纤维化概率 ≤0.594,轻度纤维化 总分对应纤维化概率 >0.594,重度纤维化 | Harrell一致性指数0.901 | |||||||||||
| Zhang, et al. (2019)[ | 体素内不相干运动参数 | 95 | 体素微循环灌注分数(f) | 组织病理学 | f ≥ 0.33,轻中度纤维化 f < 0.33,重度纤维化 | 0.876 | 92.6% | 82.4% | |||||||||
| Huang, et al. (2018)[ | T2*WI成像 | 102 | T2*WI值 | 组织病理学 | T2*WI ≥18.06 ms,轻度纤维化 T2*WI < 18.06 ms,中度或重度纤维化 | 0.951 | 94.7% | 78.3% | |||||||||
| Jordi, et al. (2015)[ | 动态对比增强成像 | 44 | 70 s至7 min增强增益百分比(%Gain) | 组织病理学 | %Gain ≤ 23.5%,无/轻度纤维化 %Gain > 23.5%,中重度纤维化 | 0.930 | 94.0% | 89.0% | |||||||||
| Li, et al. (2018)[ | 磁化传递成像 | 训练集:97 验证集:18 | 磁化传递率(MTR) | 组织病理学 | MTR ≤ 0.71,无/轻度纤维化 MTR > 0.71,中重度纤维化 | 0.919 | 84.4% | 90.0% | |||||||||
| Zhang, et al. (2024)[ | 放射组学模型 | 训练集:93 验证集:30 | ·T2WI ·增强T1WI ·DWI ·ADC ·MTI | 组织病理学 | 模型概率 ≤ 0.36,无/轻度纤维化 模型概率 > 0.36,中重度纤维化 | 0.930 | 84.0% | 100% | |||||||||
| IUS | |||||||||||||||||
| Maconi, et al. (2003)[ | Maconi 评分 | 43 | 超声下肠壁回声形态 | 组织病理学 | 低回声模式:无/轻度纤维化,以炎症为主 混合回声模式:中度纤维化,炎症和纤维化混合存在 分层回声模式:中/重度纤维化,以纤维化为主 | - | 100% | 63.3% | |||||||||
| Rispo, et al. (2017)[ | Lèmann 指数 | 71 | ·肠壁厚度 ·壁层分层 ·狭窄前扩张 ·管腔大小 | 组织病理学 | 1 级:BWT > 3.0 mm,或节段性强化而无狭窄前扩张 2 级:BWT > 4.0 mm,或肠壁分层而无狭窄前扩张 3 级:BWT > 4.0 mm,管腔狭窄,且增厚肠管上方的肠袢呈液性扩张或有回声内容物填充 | - | - | - | |||||||||
| Chen, at al.(2018)[ | 剪切波弹性成像(SWE) | 35 | SWE值 | 组织病理学 | SWE值 > 22.55 kPa,重度纤维化 SWE值 ≤ 22.55 kPa,轻/中度纤维化 | - | 69.6% | 91.7% | |||||||||
| PET-MRE | |||||||||||||||||
| Scharitzer, et al.(2023)[ | 68Ga-FAPI-PET/MRE | 14 | 最大标准摄取值(SUVmax) | 组织病理学 | SUVmax ≤ 4.4,无/轻微纤维化 4.4 < SUVmax ≤ 7.5,轻/中度纤维化 SUVmax>7.5,重度纤维化 | 0.940 | 93.0% | 83.0% | |||||||||
| [4] | XU L, HE B, SUN Y, et al. Incidence of inflammatory bowel disease in urban china: A nationwide population-based study[J]. Clin Gastroenterol Hepatol, 2023, 21(13):3379-3386.e29. |
| [5] | RIEDER F, FIOCCHI C, ROGLER G. Mechanisms, mana-gement, and treatment of fibrosis in patients with inflammatory bowel diseases[J]. Gastroenterology, 2017, 152(2):340-350.e6. |
| [6] |
LOUIS E, COLLARD A, OGER A F, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease[J]. Gut, 2001, 49(6):777-782.
doi: 10.1136/gut.49.6.777 pmid: 11709511 |
| [7] |
THIA K T, SANDBORN W J, HARMSEN W S, et al. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort[J]. Gastroenterology, 2010, 139(4):1147-1155.
doi: 10.1053/j.gastro.2010.06.070 pmid: 20637205 |
| [8] |
RIEDER F, ZIMMERMANN E M, REMZI F H, et al. Crohn's disease complicated by strictures: a systematic review[J]. Gut, 2013, 62(7):1072-1084.
doi: 10.1136/gutjnl-2012-304353 pmid: 23626373 |
| [9] | LU C, ROSENTRETER R, PARKER C E, et al. International expert guidance for defining and monitoring small bowel strictures in Crohn's disease on intestinal ultrasound: a consensus statement[J]. Lancet Gastroenterol Hepatol, 2024, 9(12):1101-1110. |
| [10] | RIEDER F, BETTENWORTH D, MA C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn's disease[J]. Aliment Pharmacol Ther, 2018, 48(3):347-357. |
| [11] | CHAN W P W, MOURAD F, LEONG R W. Crohn's disease associated strictures[J]. J Gastroenterol Hepatol, 2018, 33(5):998-1008. |
| [12] | SCHULBERG J D, WRIGHT E K, HOLT B A, et al. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn's disease strictures (STRIDENT): an open-label, single-centre, randomised controlled trial[J]. Lancet Gastroenterol Hepatol, 2022, 7(4):318-331. |
| [13] |
CHEN W, LU C, HIROTA C, et al. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn's fibrostenosing bowel strictures: A semiquantitative analysis by using a novel histological grading scheme[J]. J Crohns Colitis, 2017, 11(1):92-104.
doi: 10.1093/ecco-jcc/jjw126 pmid: 27364949 |
| [14] |
RIEDER F, LATELLA G, MAGRO F, et al. European Crohn's and Colitis Organisation Topical Review on prediction, diagnosis and management of fibrostenosing Crohn's disease[J]. J Crohns Colitis, 2016, 10(8):873-885.
doi: 10.1093/ecco-jcc/jjw055 pmid: 26928961 |
| [15] | 钟捷, 沈博, 朱维铭. 克罗恩病肠道狭窄治疗方式的选择[J]. 中华炎性肠病杂志(中英文), 2019, 3(2):169-172. |
| ZHONG J, SHEN B, ZHU W M. Choice of treatment for intestinal stenosis in Crohn's disease[J]. Chin J Inflamm Bowel Dis, 2019, 3(2):169-172. | |
| [16] |
RIEDER F, REINISCH W. Thiopurines and the natural course of Crohn's disease: did we finally find the right therapeutic target?[J]. Am J Gastroenterol, 2014, 109(7):1037-1040.
doi: 10.1038/ajg.2014.162 pmid: 24989094 |
| [17] | 中华医学会消化病学分会炎症性肠病学组, 中国炎症性肠病诊疗质量控制评估中心. 中国克罗恩病诊治指南(2023年·广州)[J]. 中华炎性肠病杂志(中英文), 2024, 8(1):2-32. |
| Inflammatory Bowel Disease Group,Chinese Society of Gastroenterology Chinese Medical Association, Inflammatory Bowel Disease Quality Control Center of China. Chinese clinical practice guideline on the management of Crohn′s disease (2023, Guangzhou)[J]. Chin J Inflamm Bowel Dis, 2024, 8(1):2-32. | |
| [18] |
RIEDER F, MUKHERJEE P K, MASSEY W J, et al. Fibrosis in IBD: from pathogenesis to therapeutic targets[J]. Gut, 2024, 73(5):854-866.
doi: 10.1136/gutjnl-2023-329963 pmid: 38233198 |
| [19] |
GORDON I O, BETTENWORTH D, BOKEMEYER A, et al. Histopathology scoring systems of stenosis associated with small bowel Crohn's disease: A systematic review[J]. Gastroenterology, 2020, 158(1):137-150.e1.
doi: S0016-5085(19)41258-4 pmid: 31476299 |
| [20] | GORDON I O, BETTENWORTH D, BOKEMEYER A, et al. International consensus to standardise histopathological scoring for small bowel strictures in Crohn's disease[J]. Gut, 2022, 71(3):479-486. |
| [21] | HUANG Q, CHEN Z, ZHANG R, et al. Intestinal fibrosis assessment in Crohn's disease patient using unenhanced spectral CT combined with 3D-printing technique[J]. Insights Imaging, 2025, 16(1):62. |
| [22] |
LI X, LIANG D, MENG J, et al. Development and validation of a novel computed-tomography enterography radiomic approach for characterization of intestinal fibrosis in Crohn's disease[J]. Gastroenterology, 2021, 160(7):2303-2316.e11.
doi: 10.1053/j.gastro.2021.02.027 pmid: 33609503 |
| [23] |
MENG J, LUO Z, CHEN Z, et al. Intestinal fibrosis classification in patients with Crohn's disease using CT enterography-based deep learning: comparisons with radiomics and radiologists[J]. Eur Radiol, 2022, 32(12):8692-8705.
doi: 10.1007/s00330-022-08842-z pmid: 35616733 |
| [24] | LI X H, FENG S T, CAO Q H, et al. Degree of creeping fat assessed by computed tomography enterography is associated with intestinal fibrotic stricture in patients with Crohn's disease: A potentially novel mesenteric cree-ping fat index[J]. J Crohns Colitis, 2021, 15(7):1161-1173. |
| [25] | MENG J, MAO Y, ZHOU J, et al. Mesenteric abnormalities play an important role in grading intestinal fibrosis in patients with Crohn's disease: a computed tomography and clinical marker-based nomogram[J]. Therap Adv Gastroenterol, 2022, 15:17562848221122504. |
| [26] | LI X H, MAO R, HUANG S Y, et al. Ability of DWI to characterize bowel fibrosis depends on the degree of bowel inflammation[J]. Eur Radiol, 2019, 29(5):2465-2473. |
| [27] |
CARUSO A, ANGRIMAN I, SCARPA M, et al. Diffusion-weighted magnetic resonance for assessing fibrosis in Crohn's disease[J]. Abdom Radiol (NY), 2020, 45(8):2327-2335.
doi: 10.1007/s00261-019-02167-0 pmid: 31392397 |
| [28] | COIMBRA A, RIMOLA J, CUATRECASAS M, et al. Magnetic resonance enterography and histology in patients with fibrostenotic Crohn's disease: A multicenter study[J]. Clin Transl Gastroenterol, 2022, 13(7):e00505. |
| [29] | DU J F, LU B L, HUANG S Y, et al. A novel identification system combining diffusion kurtosis imaging with conventional magnetic resonance imaging to assess intestinal strictures in patients with Crohn's disease[J]. Abdom Radiol (NY), 2021, 46(3):936-947. |
| [30] | 杜金芳, 黄丽, 毛弈涛, 等. 基于MRI扩散峰度成像的列线图预测克罗恩病肠壁纤维化的研究[J]. 中华放射学杂志, 2020, 54(8):792-798. |
| DU J F, HUANG L, MAO Y T, et al. A diffusion kurtosis imaging based nomogram for assessment of bowel fibrosis in patients with Crohn disease[J]. Chin J Radiol, 2020, 54(8):792-798. | |
| [31] | ZHANG M C, LI X H, HUANG S Y, et al. IVIM with fractional perfusion as a novel biomarker for detecting and grading intestinal fibrosis in Crohn's disease[J]. Eur Radiol, 2019, 29(6):3069-3078. |
| [32] | HUANG S Y, LI X H, HUANG L, et al. T2* Mapping to characterize intestinal fibrosis in crohn's disease[J]. J Magn Reson Imaging. |
| [33] |
RIMOLA J, PLANELL N, RODRÍGUEZ S, et al. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging[J]. Am J Gastroenterol, 2015, 110(3):432-440.
doi: 10.1038/ajg.2014.424 pmid: 25623654 |
| [34] |
ADLER J, SWANSON S D, SCHMIEDLIN-REN P, et al. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease[J]. Radiology, 2011, 259(1):127-135.
doi: 10.1148/radiol.10091648 pmid: 21324841 |
| [35] | LI X H, MAO R, HUANG S Y, et al. Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging[J]. Radiology, 2018, 287(2):494-503. |
| [36] | ZHANG M, ZENG Y, FANG Z N, et al. MRI radiomics enhances radiologists' ability for characterizing intestinal fibrosis in patients with Crohn's disease[J]. Insights Ima-ging, 2024, 15(1):165. |
| [37] | LU C, ROSENTRETER R, DELISLE M, et al. Systematic review: Defining, diagnosing and monitoring small bowel strictures in Crohn's disease on intestinal ultrasound[J]. Aliment Pharmacol Ther, 2024, 59(8):928-940. |
| [38] | BHATNAGAR G, RODRIGUEZ-JUSTO M, HIGGINSON A, et al. Inflammation and fibrosis in Crohn's disease: location-matched histological correlation of small bowel ultrasound features[J]. Abdom Radiol (NY), 2021, 46(1):144-155. |
| [39] |
RIPOLLÉS T, RAUSELL N, PAREDES J M, et al. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn's disease: a comparison with surgical histopathology analysis[J]. J Crohns Colitis, 2013, 7(2):120-128.
doi: 10.1016/j.crohns.2012.03.002 pmid: 22483566 |
| [40] |
NYLUND K, JIRIK R, MEZL M, et al. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn's disease[J]. Ultrasound Med Biol, 2013, 39(7):1197-1206.
doi: 10.1016/j.ultrasmedbio.2013.01.020 pmid: 23643057 |
| [41] |
LU C, GUI X, CHEN W, et al. Ultrasound shear wave elastography and contrast enhancement: effective biomarkers in Crohn's disease strictures[J]. Inflamm Bowel Dis, 2017, 23(3):421-430.
doi: 10.1097/MIB.0000000000001020 pmid: 28129289 |
| [42] | DING S S, FANG Y, WAN J, et al. Usefulness of Strain elastography, ARFI Imaging, and point shear wave elastography for the assessment of Crohn disease strictures[J]. J Ultrasound Med, 2019, 38(11):2861-2870. |
| [43] | MACONI G, CARSANA L, FOCIANI P, et al. Small bowel stenosis in Crohn's disease: clinical, biochemical and ultrasonographic evaluation of histological features[J]. Aliment Pharmacol Ther, 2003, 18(7):749-756. |
| [44] | PARIENTE B, MARY J Y, DANESE S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn's disease[J]. Gastroentero-logy, 2015, 148(1):52-63.e3. |
| [45] |
RISPO A, IMPERATORE N, TESTA A, et al. Bowel damage in Crohn's disease: direct comparison of ultrasonography-based and magnetic resonance-based lemann index[J]. Inflamm Bowel Dis, 2017, 23(1):143-151.
doi: 10.1097/MIB.0000000000000980 pmid: 27930407 |
| [46] | CHEN Y J, MAO R, LI X H, et al. Real-time shear wave ultrasound elastography differentiates fibrotic from inflammatory strictures in patients with Crohn's disease[J]. Inflamm Bowel Dis, 2018, 24(10):2183-2190. |
| [47] |
LU C, MERRILL C, MEDELLIN A, et al. Bowel ultrasound state of the art: grayscale and Doppler ultrasound, contrast enhancement, and elastography in Crohn disease[J]. J Ultrasound Med, 2019, 38(2):271-288.
doi: 10.1002/jum.14920 pmid: 30604884 |
| [48] | FRAQUELLI M, SARNO A, GIRELLI C, et al. Reprodu-cibility of bowel ultrasonography in the evaluation of Crohn's disease[J]. Dig Liver Dis, 2008, 40(11):860-866. |
| [49] |
LOUIS E, ANCION G, COLARD A, et al. Noninvasive assessment of Crohn's disease intestinal lesions with (18)F-FDG PET/CT[J]. J Nucl Med, 2007, 48(7):1053-1059.
doi: 10.2967/jnumed.107.040436 pmid: 17574978 |
| [50] |
CATALANO O A, GEE M S, NICOLAI E, et al. Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease[J]. Radiology, 2016, 278(3):792-800.
doi: 10.1148/radiol.2015150566 pmid: 26436860 |
| [51] | PELLINO G, NICOLAI E, CATALANO O A, et al. PET/MR versus PET/CT imaging: impact on the clinical mana-gement of small-bowel Crohn's disease[J]. J Crohns Colitis, 2016, 10(3):277-285. |
| [52] | PAN Q, XU H, LIU S, et al. Head-to-head comparison of 68Ga-FAPI-04 and 18F-FDG PET/CT for the assessment of Crohn's disease : a prospective pilot study[J]. Clin Nucl Med, 2025, 50(6):473-479. |
| [53] | LI Z, CHEN Z, ZHANG R, et al. Comparative analysis of [18F]F-FAPI PET/CT, [18F]F-FDG PET/CT and magnetization transfer MR imaging to detect intestinal fibrosis in Crohn's disease: A prospective animal model and human cohort study[J]. Eur J Nucl Med Mol Imaging, 2024, 51(7):1856-1868. |
| [54] | SCHARITZER M, MACHER-BEER A, MANG T, et al. Evaluation of Intestinal fibrosis with 68Ga-FAPI PET/MR enterography in Crohn disease[J]. Radiology, 2023, 307(3):e222389. |
| [55] | 中国炎症性肠病诊疗质量控制评估中心, 中华医学会消化病学分会炎症性肠病学组, 中华医学会超声医学分会腹部超声学组, 等. 中国炎症性肠病肠道超声检查和报告规范专家指导意见[J]. 胃肠病学, 2024, 29(5):283-290. |
| [1] |
TORRES J, MEHANDRU S, COLOMBEL J F, et al. Crohn's disease[J]. Lancet, 2017, 389(10080):1741-1755.
doi: S0140-6736(16)31711-1 pmid: 27914655 |
| [2] |
D'HAENS G, RIEDER F, FEAGAN B G, et al. Challenges in the pathophysiology, diagnosis, and management of intestinal fibrosis in inflammatory bowel disease[J]. Gastroenterology, 2022, 162(1):26-31.
doi: 10.1053/j.gastro.2019.05.072 pmid: 31254502 |
| [3] | NG S C, SHI H Y, HAMIDI N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies[J]. Lancet, 2017, 390(10114):2769-2778. |
| [55] | Inflammatory Bowel Disease Quality Control Center of China, Inflammatory Bowel Disease Group, Chinese So-ciety of Gastroenterology, Chinese Medical Association; Abdominal Ultrasonography Group, Chinese Society of Ultrasonography, Chinese Medical Association. et al. Experts' suggestions on standardization of intestinal ultrasound examination and reporting for inflammatory bowel disease in China[J]. Chin J Gastroenterol, 2024, 29(5):283-290. |
| [56] | 李雪华, 冯仕庭, 黄丽, 等. 中国炎症性肠病影像检查及报告规范专家指导意见[J]. 中华炎性肠病杂志, 2021, 5(2):109-113. |
| LI X H, FENG S T, HUANG L, et al. Expert guideline on imaging examination and report specification of inflammatory bowel disease in China[J]. Chin J Inflamm Bowel Dis, 2021, 5(2):109-113. |
| [1] | WU Shuang, XIE Qian, GUAN Xueni, ZHANG Sufang, GAO Xinfang, LIANG Zonghui. Perfomence of MRI intravoxel incoherent motion diffusion weighted imaging parameters in diagnosing active Crohn's disease [J]. Journal of Diagnostics Concepts & Practice, 2020, 19(02): 157-161. |
| [2] | YU Youyou, ZENG Junxiang, LUO Ting, DENG Lin, PAN Xiujun. Comparison of results and evaluation of performance of three different ELISA kits for detection of ASCA [J]. Journal of Diagnostics Concepts & Practice, 2019, 18(04): 454-459. |
| [3] | WANG Tingting, ZHENG Naisheng, YUAN Xiangliang, SHEN Lisong. Analysis of structural characteristics of gut microbiome in colitis mice based on 16S rRNA high-throughput sequencing [J]. Journal of Diagnostics Concepts & Practice, 2019, 18(03): 263-270. |
| [4] | WANG Weiyi, ZHANG Yongping, YUAN Yaozong, WU Yunlin, CHEN Ping. Experimental study on effect of PIAS1 in regulating migration of macrophage and its mechanism [J]. Journal of Diagnostics Concepts & Practice, 2017, 16(01): 60-65. |
| [5] | MAO Yulei, ZHOU Tao, TANG Lingyun, ZHANG Hongxin, WANG Zhugang. Influence of Tacr2 on ulcerative colitis in mice [J]. Journal of Diagnostics Concepts & Practice, 2016, 15(06): 578-581. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||